Microstructure and characteristics of phase-separated gels of Type-A gelatin and hydroxypropyl starch: pH responsiveness
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The impact of phase separation behavior on the performance of hydrogels has always been the research focus. This study explored the relationship of “pH-microstructure-hydrogel properties” of phase-separated hydrogels composed of type-A gelatin (GE, 5 wt%) and hydroxypropyl starch (HPS, 3 wt%). The results revealed that changes in the microstructure of the hydrogel at different pHs significantly impacted the gel properties. At pH 4.00 and 11.00, phase separation occurred in the GE/HPS gel with a microstructure of HPS-in-GE. In particular, at pH 5.00, 6.00, 7.00, and 9.00, severe phase separation resulted in the separation of substantial aggregates of HPS and GE, resulting in a continuous phase structure. At pH 2.00 and 3.00, phase separation was suppressed, resulting in a homogeneous microstructure. Compared to the phase-separated gels at pH 4.00–11.00, the homogeneous systems at pH 2.00 and 3.00 displayed a synergistic effect with higher gel strength. Analysis of intermolecular forces in the GE/HPS gel indicated that hydrogen bonding was the primary interaction force. Furthermore, the addition of GE/HPS into hawthorn jelly at about pH 3.00 effectively preserved the structural appearance and exhibited higher levels of hardness, thus improving the sensory properties of hawthorn jelly. In summary, phase separation decreased the storage modulus of GE/HPS gel, but the compatibility of GE/HPS macromolecules exhibited synergistic effects at 2.00 and 3.00 and improved the hydrogel mechanical properties. This work provides some new insights for GE/HPS-based gel foods.
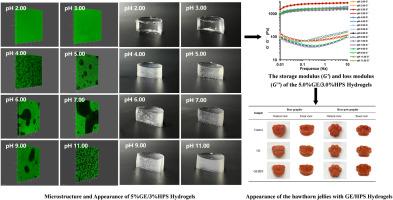
A型明胶和羟丙基淀粉相分离凝胶的微观结构和特性:对pH值的响应性
相分离行为对水凝胶性能的影响一直是研究的重点。本研究探讨了由 A 型明胶(GE,5 wt%)和羟丙基淀粉(HPS,3 wt%)组成的相分离水凝胶的 "pH 值-微观结构-水凝胶性能 "关系。结果表明,在不同的 pH 值下,水凝胶微观结构的变化对凝胶特性有显著影响。在 pH 值为 4.00 和 11.00 时,GE/HPS 凝胶中出现了相分离现象,其微观结构为 HPS-in-GE。特别是在 pH 值为 5.00、6.00、7.00 和 9.00 时,严重的相分离导致 HPS 和 GE 的大量聚集体分离,形成连续的相结构。在 pH 值为 2.00 和 3.00 时,相分离受到抑制,形成了均匀的微观结构。与 pH 值为 4.00-11.00 的相分离凝胶相比,pH 值为 2.00 和 3.00 的均相体系具有协同效应,凝胶强度更高。对 GE/HPS 凝胶中分子间作用力的分析表明,氢键是主要的相互作用力。此外,在 pH 值为 3.00 左右的山楂果冻中加入 GE/HPS 后,可有效保持结构外观,并表现出更高的硬度,从而改善山楂果冻的感官特性。总之,相分离降低了 GE/HPS 凝胶的储存模量,但 GE/HPS 大分子的相容性在 2.00 和 3.00 时表现出协同效应,改善了水凝胶的机械性能。这项工作为基于 GE/HPS 的凝胶食品提供了一些新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


