Capacitive lithium capture system using a mixed LiMn2O4 and LiAlO2 material
IF 8.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The increasing demand for lithium (Li), a crucial material in various industries, requires efficient recovery methods and a shift toward a circular economy. This study investigates a fast, eco-friendly technique for selective Li recovery, emphasizing the use of innovative materials from spent Li-ion batteries (SLiBs), particularly LiMn2O4(LMO)/LiAlO2(LAO)-based materials, to enhance Li's circular economy. Conventional Li recovery methods typically involve prolonged processes with chemical additives and environmental concerns, whereas electrochemical systems like membrane-based capacitive deionization (MCDI) offer promising high removal capacities, regeneration ability, and scalability. However, no commercial electrochemical Li recovery system underscores the need for continued research to improve their performance. This study employs MCDI for selective Li recovery, examining various electrode materials, including commercial activated carbon, LMO-based electrodes, and modified LMO/LAO-based electrodes. The mixed LiMn2O4/LiAlO2 cathode exhibited high selectivity for Li+ extraction with a recovery efficiency of 83.1 %, achieving a deionization capacity of 38.15 mg/g at 1.0 V under an initial feed concentration of 5 mM LiCl. The Li+ adsorption reached 900 μmol/g, with a separation factor ( of 3.77 (CMg2+/CLi+ = 1), setting a robust foundation for a comprehensive Li recovery framework that meets the increasing Li demand while minimizing environmental impact.
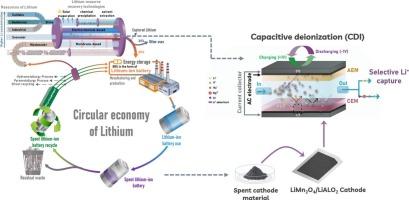
使用 LiMn2O4 和 LiAlO2 混合材料的电容式锂捕获系统
锂(Li)是各行各业的重要材料,其需求量不断增加,这就要求采用高效的回收方法,并向循环经济转变。本研究调查了一种快速、环保的选择性锂回收技术,强调使用废旧锂离子电池(SLiBs)中的创新材料,特别是基于锰酸锂(LMO)/铝酸锂(LAO)的材料,以提高锂的循环经济性。传统的锂回收方法通常涉及使用化学添加剂的漫长过程和环境问题,而膜法电容去离子(MCDI)等电化学系统则具有高去除能力、再生能力和可扩展性等优点。然而,目前还没有商业化的电化学锂回收系统,因此需要继续研究以提高其性能。本研究采用 MCDI 技术进行选择性锂回收,研究了各种电极材料,包括商用活性炭、基于 LMO 的电极和基于 LMO/LAO 的改性电极。LiMn2O4/LiAlO2 混合阴极对 Li+ 的提取具有高选择性,回收效率高达 83.1%,在初始进料浓度为 5 mM LiCl 的条件下,1.0 V 下的去离子容量为 38.15 mg/g。对 Li+ 的吸附量达到 900 μmol/g,分离因子(αMg2+Li+)为 3.77(CMg2+/CLi+ = 1),为建立全面的锂回收框架奠定了坚实的基础,从而在满足日益增长的锂需求的同时,最大限度地减少对环境的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


