Degradation of ciprofloxacin by hydrogen peroxide activated with pyrite under simulated sunlight
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
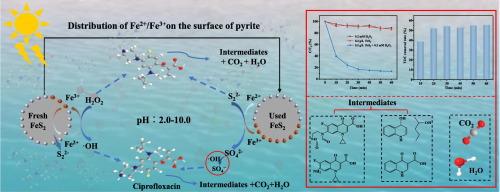
Ciprofloxacin (CIP) residues in the environment pose risks to both human health and ecosystems. In this study, we explored the effect of hydrogen peroxide (H2O2) on CIP degradation, activated by pyrite (FeS2) under simulated sunlight. XRD, XRF, EDS, XSP, DRS, and PL tests confirmed the high purity of FeS2 and its photocatalytic properties. With [CIP] = 30 μM, [FeS2] = 0.2 g/L, [H2O2] = 0.2 mM, CIP removal reached 87.6 %. The removal rate of TOC reached 55.3 % after 60 min of light exposure, and hydroxyl radicals (![]() OH) contributed 72.7 % to the process. H2O2 utilization was 67.5 %, and the system operated effectively across a pH range of 2.00 to 8.00. CIP removal in river water reached 87.9 % after 3 h of light exposure, though the degradation was slower than in ultrapure water. Cl−, SO42−, and NO3− had little effect on degradation, whereas H2PO4−, CO32−, and HCO3− significantly inhibited the process as their concentrations increased. In livestock wastewater, the simulated sunlight-FeS2/H2O2 system was less effective, but after diluting the wastewater 100 times, CIP removal reached 87.6 % after 60 min. The degraded solutions showed no significant toxicity. These findings suggest that FeS2, combined with simulated sunlight, can effectively catalyze low concentrations of H2O2 to produce
OH) contributed 72.7 % to the process. H2O2 utilization was 67.5 %, and the system operated effectively across a pH range of 2.00 to 8.00. CIP removal in river water reached 87.9 % after 3 h of light exposure, though the degradation was slower than in ultrapure water. Cl−, SO42−, and NO3− had little effect on degradation, whereas H2PO4−, CO32−, and HCO3− significantly inhibited the process as their concentrations increased. In livestock wastewater, the simulated sunlight-FeS2/H2O2 system was less effective, but after diluting the wastewater 100 times, CIP removal reached 87.6 % after 60 min. The degraded solutions showed no significant toxicity. These findings suggest that FeS2, combined with simulated sunlight, can effectively catalyze low concentrations of H2O2 to produce ![]() OH radicals, removing antibiotics from livestock wastewater.
OH radicals, removing antibiotics from livestock wastewater.
过氧化氢在模拟阳光下活化黄铁矿降解环丙沙星
环境中的环丙沙星(CIP)残留物对人类健康和生态系统都构成了风险。在本研究中,我们探讨了过氧化氢(H2O2)在模拟阳光下由黄铁矿(FeS2)激活对 CIP 降解的影响。XRD、XRF、EDS、XSP、DRS 和 PL 测试证实了 FeS2 的高纯度及其光催化特性。在 [CIP] = 30 μM、[FeS2] = 0.2 g/L、[H2O2] = 0.2 mM 的条件下,CIP 的去除率达到 87.6%。光照 60 分钟后,TOC 的去除率达到 55.3%,羟基自由基(OH)对该过程的贡献率为 72.7%。H2O2 的利用率为 67.5%,系统可在 2.00 至 8.00 的 pH 值范围内有效运行。光照 3 小时后,河水中 CIP 的去除率达到 87.9%,但降解速度比超纯水慢。Cl-、SO42- 和 NO3- 对降解的影响很小,而 H2PO4-、CO32- 和 HCO3- 则会随着浓度的增加而明显抑制降解过程。在畜牧业废水中,模拟阳光-FeS2/H2O2 系统的效果较差,但将废水稀释 100 倍后,60 分钟后 CIP 的去除率达到 87.6%。降解后的溶液没有显示出明显的毒性。这些研究结果表明,FeS2 与模拟阳光相结合,可有效催化低浓度 H2O2 产生 OH 自由基,从而去除畜牧业废水中的抗生素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


