Ni-CoS2 nanoparticles loaded on 3D RGO for efficient electrochemical hydrogen and oxygen evolution reaction
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The development of efficient non-precious metal electrocatalysts for electrochemical water splitting is still a huge challenge. In this study, we designed and synthesized an efficient electrocatalyst for Ni-doped cobalt sulfide supported on 3D RGO (Ni-CoS2/3D RGO) using a simple one-step solvent-thermal method. Ni doping adjusted the charge distribution on the surface of the material, significantly improved the catalytic activity, and then accelerated the reaction kinetics. The high specific surface area and high stability of 3D RGO greatly improved the intrinsic activity of the material, making Ni-CoS2/3D RGO exhibit superior catalytic activity in both electrochemical hydrogen evolution and oxygen evolution. We evaluated the morphology and properties of the catalysts through a series of characterization methods and electrochemical performance tests. When the current density is 10 mA cm−2, the HER overpotential of Ni-CoS2/3D RGO under acidic condition reaches 138 mV, and the Tafel slope is 61 mV dec−1. Under alkaline conditions, the OER overpotential reaches 286 mV, and the Tafel slope is only 48 mV dec−1. And the OWS overpotential of the catalyst is 1.41 V and 1.82 V under acidic and alkaline conditions, respectively, indicating that the catalyst has ideal water splitting performance. This work provides a new idea for the application of 3D reduced graphene oxide in electrochemical direction, and also provides a new strategy for the design and preparation of non-precious metal catalysts for the efficient electrochemical water splitting.
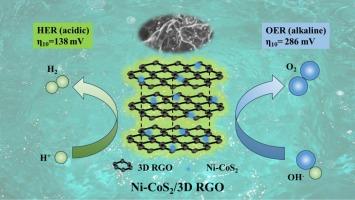
负载在三维 RGO 上的 Ni-CoS2 纳米粒子用于高效的电化学氢氧进化反应
开发用于电化学水分离的高效非贵金属电催化剂仍然是一个巨大的挑战。在本研究中,我们采用简单的一步溶剂热法设计并合成了一种在三维 RGO(Ni-CoS2/3D RGO)上支撑的掺镍硫化钴高效电催化剂。掺杂镍调整了材料表面的电荷分布,显著提高了催化活性,进而加速了反应动力学。三维 RGO 的高比表面积和高稳定性大大提高了材料的内在活性,使 Ni-CoS2/3D RGO 在电化学氢进化和氧进化中都表现出卓越的催化活性。我们通过一系列表征方法和电化学性能测试评估了催化剂的形态和性质。当电流密度为 10 mA cm-2 时,Ni-CoS2/3D RGO 在酸性条件下的氢进化过电位达到 138 mV,Tafel 斜率为 61 mV dec-1。在碱性条件下,OER 过电位达到 286 mV,Tafel 斜率仅为 48 mV dec-1。在酸性和碱性条件下,催化剂的 OWS 过电位分别为 1.41 V 和 1.82 V,表明催化剂具有理想的水分离性能。这项工作为三维还原氧化石墨烯在电化学方向的应用提供了新思路,也为设计和制备非贵金属催化剂实现高效电化学分水提供了新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


