Design and implementation of an accelerating rate calorimeter based on Modelica modeling
IF 3.1
2区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The safety of self-reactive chemicals has garnered attention due to their immense destructive power. The use of an accelerating rate calorimeter allows for more accurate measurement and understanding of the kinetic parameters and reaction mechanisms of self-reactive chemicals, thereby reducing the occurrence of major accidents. In this study, a new accelerating rate calorimeter is designed and constructed by combining Modelica and a fuzzy Proportional-Integral-Derivative algorithm. The feasibility of the calorimeter data was verified by using two solutions with significantly different reaction rates: 20 % mass fraction di‑tert‑butyl peroxide/toluene and tert-butylperoxy-2-ethylhecanoate. Their thermal hazard characteristic parameters were compared with the literature data. In addition, the risk level of thermal runaway was determined using the Stoessel risk assessment method. These results demonstrate that the accelerating rate calorimeter based on Modelica modeling meets the accuracy of thermal hazard characteristic parameters. It is capable of performing risk assessments for runaway reactions of self-reactive chemicals.
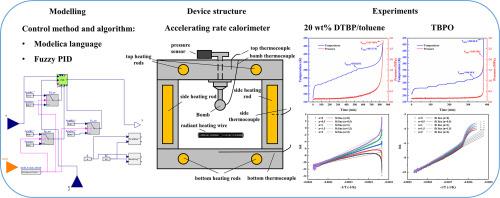
基于 Modelica 建模的加速热量计的设计与实施
自反应化学品具有巨大的破坏力,因此其安全性备受关注。使用加速量热仪可以更准确地测量和了解自反应化学品的动力学参数和反应机理,从而减少重大事故的发生。在本研究中,通过结合 Modelica 和模糊比例-积分-求导算法,设计并构建了一种新的加速速率量热仪。通过使用两种反应速率明显不同的解决方案,验证了量热计数据的可行性:质量分数为 20% 的过氧化二叔丁基/甲苯和过氧化叔丁基-2-乙基己酸酯。它们的热危害特征参数与文献数据进行了比较。此外,还采用斯托塞尔风险评估方法确定了热失控的风险等级。这些结果表明,基于 Modelica 建模的加速热量计能够满足热危害特征参数的准确性要求。它能够对自反应化学品的失控反应进行风险评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Thermochimica Acta
化学-分析化学
CiteScore
6.50
自引率
8.60%
发文量
210
审稿时长
40 days
期刊介绍:
Thermochimica Acta publishes original research contributions covering all aspects of thermoanalytical and calorimetric methods and their application to experimental chemistry, physics, biology and engineering. The journal aims to span the whole range from fundamental research to practical application.
The journal focuses on the research that advances physical and analytical science of thermal phenomena. Therefore, the manuscripts are expected to provide important insights into the thermal phenomena studied or to propose significant improvements of analytical or computational techniques employed in thermal studies. Manuscripts that report the results of routine thermal measurements are not suitable for publication in Thermochimica Acta.
The journal particularly welcomes papers from newly emerging areas as well as from the traditional strength areas:
- New and improved instrumentation and methods
- Thermal properties and behavior of materials
- Kinetics of thermally stimulated processes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


