Optimization of electrochemical oxidation for the degradation of acid yellow 99 via sulfate and hydroxyl radicals activation: A study using Plackett–Burman and Box–Behnken designs
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
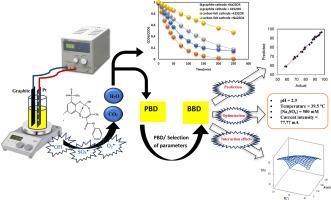
The synthetic azo dye acid yellow 99 (AY99) is a nonbiodegradable and carcinogenic pollutant widely used in dyeing paper, leather, textiles, protein fibers, and other industries. The degradation of AY99 in an aqueous solution by the electrochemical oxidation process EOP over a platinum grid anode and graphite cathode was studied. The results indicated that the degradation of AY 99 by the EOP process followed second-order reaction kinetics (R2 > 0.98). To optimize a range of operating parameters, such as pH (1–4), temperature (20–40 °C), electrolyte concentration (50–500 mM), supporting electrolyte species (Na2SO4, NaCl), applied current (60–500 mA), initial concentration of AY99 (0.05–0.2 mM), Plackett–Burman design and Box–Behnken statistical experimental design (BBD) were applied. The proposed quadratic model was validated by a high adjusted correlation coefficient (R2adj = 0.987) and a high correlation coefficient (R2 = 0.994). The order of the main factors affecting the degradation efficiency was as follows: pH > current intensity > [Na2SO4] > temperature. On the basis of the optimization results, a maximum removal efficiency > 98 % was obtained under the following conditions: pH = 2.9, temperature = 39.5 °C, [Na2SO4] = 500 mM, and current intensity = 77.77 mA. Comparative mineralization processes with different cathode materials (carbon felt and graphite) and various electrolyte concentrations were also studied under optimum conditions. This study demonstrated that complete removal of AY 99 could be achieved through this electrochemical oxidation (Pt anode/graphite cathode/(Na2SO4 or K2S2O8) electrolytes) by sulfate (SO4![]() −) and hydroxyl (
−) and hydroxyl (![]() OH) radicals generated during the process.
OH) radicals generated during the process.
通过硫酸根和羟基自由基活化优化电化学氧化法降解酸性黄 99:使用普拉克特-伯曼设计和方框-贝肯设计进行的研究
合成偶氮染料酸性黄 99(AY99)是一种不可生物降解的致癌污染物,广泛用于纸张、皮革、纺织品、蛋白质纤维等行业的染色。研究人员在铂栅阳极和石墨阴极上采用电化学氧化工艺 EOP 对水溶液中的 AY99 进行了降解。结果表明,EOP 过程对 AY99 的降解遵循二阶反应动力学(R2 > 0.98)。为了优化一系列操作参数,如 pH 值(1-4)、温度(20-40 °C)、电解质浓度(50-500 mM)、支持电解质种类(Na2SO4、NaCl)、应用电流(60-500 mA)、AY99 初始浓度(0.05-0.2 mM),应用了普拉克特-伯曼设计和箱-贝肯统计实验设计(BBD)。通过高调整相关系数(R2adj = 0.987)和高相关系数(R2 = 0.994)验证了所提出的二次模型。影响降解效率的主要因素顺序为:pH 值;电流强度;[Na2SO4] ;温度。根据优化结果,在 pH = 2.9、温度 = 39.5 °C、[Na2SO4] = 500 mM 和电流强度 = 77.77 mA 的条件下,获得了最高去除率 98%。在最佳条件下,还研究了不同阴极材料(碳毡和石墨)和不同电解质浓度的矿化过程比较。该研究表明,通过这种电化学氧化(铂阳极/石墨阴极/(Na2SO4 或 K2S2O8)电解质)过程中产生的硫酸根(SO4-)和羟基(OH)自由基,可以完全去除 AY 99。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


