La-Al modified Fe3O4 adsorbent for adsorption performance and mechanism of low P concentration in water
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
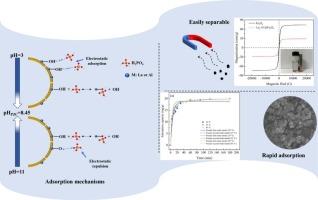
Adsorbents of La![]() Al hydroxide (oxygen) species have been widely used for removal of phosphate (P) from water, but fewer studies were conducted on the removal of low P concentration. This study firstly fabricated a new composite material of La
Al hydroxide (oxygen) species have been widely used for removal of phosphate (P) from water, but fewer studies were conducted on the removal of low P concentration. This study firstly fabricated a new composite material of La![]() Al modified Fe3O4 by co-precipitation method applied for removal of low P concentration in water. Results showed that the optimal adsorbent performance was at a La/Al molar ratio of 2:1 and an optimum dosage of 0.1 g/L. P removal was >99 % with a low initial P concentration (2 mg/L), and the residual P was only 0.02 mg/L. La2-Al@Fe3O4 adsorbent was an irregular magnetic particle with a large specific surface area of 101.35 m2/g. Adsorption process primarily involved chemisorption and formation of multiple adsorption layers. The maximum adsorption capacity was 71.75 mg/g when the initial P concentration was 50 mg/L. Meanwhile, it showed good adsorption performance at pH 4.0–9.0, exhibited notable selectivity for P despite competing ions such as Cl−, HCO3−, SO42−, and NO3−, and was influenced by coexisting citric acid and humic acid. Also, La2-Al@Fe3O4 showed good reproducibility and maintained 85.6 % of the original adsorption capacity after five adsorption and desorption cycles. Additionally, P concentration could be reduced to below 0.02 mg/L in actual water, and La2-Al@Fe3O4 was friendly to the water environment based on the toxicity analysis of Chlorella vulgaris. The adsorption mechanism of P by La2-Al@Fe3O4 mainly involved electrostatic adsorption and ligand exchange. This novel adsorbent shows great potential for application in low P concentration water treatment.
Al modified Fe3O4 by co-precipitation method applied for removal of low P concentration in water. Results showed that the optimal adsorbent performance was at a La/Al molar ratio of 2:1 and an optimum dosage of 0.1 g/L. P removal was >99 % with a low initial P concentration (2 mg/L), and the residual P was only 0.02 mg/L. La2-Al@Fe3O4 adsorbent was an irregular magnetic particle with a large specific surface area of 101.35 m2/g. Adsorption process primarily involved chemisorption and formation of multiple adsorption layers. The maximum adsorption capacity was 71.75 mg/g when the initial P concentration was 50 mg/L. Meanwhile, it showed good adsorption performance at pH 4.0–9.0, exhibited notable selectivity for P despite competing ions such as Cl−, HCO3−, SO42−, and NO3−, and was influenced by coexisting citric acid and humic acid. Also, La2-Al@Fe3O4 showed good reproducibility and maintained 85.6 % of the original adsorption capacity after five adsorption and desorption cycles. Additionally, P concentration could be reduced to below 0.02 mg/L in actual water, and La2-Al@Fe3O4 was friendly to the water environment based on the toxicity analysis of Chlorella vulgaris. The adsorption mechanism of P by La2-Al@Fe3O4 mainly involved electrostatic adsorption and ligand exchange. This novel adsorbent shows great potential for application in low P concentration water treatment.
La-Al 改性 Fe3O4 吸附剂对水中低浓度 P 的吸附性能和机理研究
LaAl 氢氧化物(氧)吸附剂已被广泛用于去除水中的磷酸盐,但对去除低浓度磷酸盐的研究较少。本研究首次采用共沉淀法制备了一种新型 LaAl 改性 Fe3O4 复合材料,用于去除水中的低浓度磷酸盐。结果表明,当 La/Al 摩尔比为 2:1、最佳用量为 0.1 g/L 时,吸附剂的性能最佳。在初始 P 浓度较低(2 mg/L)的情况下,P 的去除率为 99%,残余 P 仅为 0.02 mg/L。La2-Al@Fe3O4 吸附剂是一种不规则的磁性颗粒,具有 101.35 m2/g 的大比表面积。吸附过程主要涉及化学吸附和多吸附层的形成。当初始 P 浓度为 50 mg/L 时,最大吸附容量为 71.75 mg/g。同时,它在 pH 值为 4.0-9.0 的条件下表现出良好的吸附性能,尽管有 Cl-、HCO3-、SO42- 和 NO3-等竞争离子的存在,但对 P 仍有显著的选择性,并受到柠檬酸和腐殖酸共存的影响。此外,La2-Al@Fe3O4 还表现出良好的重现性,经过五个吸附和解吸周期后,其吸附容量仍保持在原来的 85.6%。此外,La2-Al@Fe3O4 可将实际水中的 P 浓度降至 0.02 mg/L 以下,而且根据对绿藻的毒性分析,La2-Al@Fe3O4 对水环境友好。La2-Al@Fe3O4 对 P 的吸附机理主要涉及静电吸附和配体交换。这种新型吸附剂在低 P 浓度水处理中具有巨大的应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


