Intra-BLA alteration of interneurons’ modulation of activity in rats, reveals a dissociation between effects on anxiety symptoms and extinction learning
IF 3.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
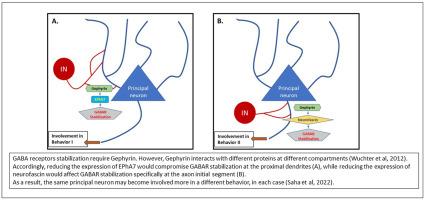
The basolateral amygdala (BLA) is a dynamic brain region involved in emotional experiences and subject to long-term plasticity. The BLA also modulates activity, plasticity, and related behaviors associated with other brain regions, including the mPFC and hippocampus. Accordingly, intra-BLA plasticity can be expected to alter both BLA-dependent behaviors and behaviors mediated by other brain regions. Lasting intra-BLA plasticity may be considered a form of metaplasticity, since it will affect subsequent plasticity and response to challenges later on. Activity within the BLA is tightly modulated by GABAergic interneurons, and thus inducing lasting alteration of GABAergic modulation of principal neurons may have an impactful metaplastic effect on BLA functioning. Previously, we demonstrated that intra-BLA knockdown (KD) of neurofascin (NF) reduced GABAergic synapses exclusively at the axon initial segment (AIS). Here, by reducing the expression of the tyrosine kinase receptor ephrin A7 (EphA7), we selectively impaired the modulatory function of a different subpopulation of interneurons, specifically targeting the soma and proximal dendrites of principal neurons. This perturbation induced an expected reduction in the spontaneous inhibitory synaptic input and an increase in the excitatory spontaneous synaptic activity, most probably due to the reduction of inhibitory tone. Moreover, this increased synaptic activity was followed by a reduction in intrinsic excitability. While intra-BLA NF-KD resulted in impaired extinction learning, without increased symptoms of anxiety, intra-BLA reduction of EphA7 expression resulted in increased symptoms of anxiety, as measured in the elevated plus maze, but without affecting fear conditioning or extinction learning. These results confirm the role of the BLA and intra-BLA metaplasticity in stress-induced increased anxiety symptoms and in impaired fear extinction learning but reveals a difference in intra-BLA mechanisms involved. The results also confirm the contribution of GABAergic interneurons to these effects but indicate selective roles for different subpopulations of intra-BLA interneurons.
大鼠神经元间活动调节的 BLA 内改变揭示了对焦虑症状和消退学习的不同影响
杏仁基底外侧(BLA)是一个动态脑区,参与情绪体验并具有长期可塑性。基底外侧杏仁核还能调节其他脑区的活动、可塑性和相关行为,包括前脑皮质和海马。因此,BLA内部的可塑性可望改变依赖于BLA的行为和由其他脑区介导的行为。BLA内的持久可塑性可被视为一种元可塑性,因为它将影响以后的可塑性和对挑战的反应。BLA内的活动受到GABA能中间神经元的严格调控,因此诱导持久改变GABA能对主要神经元的调控可能会对BLA的功能产生影响。此前,我们曾证实,在BLA内敲除(KD)神经瀑蛋白(NF)会减少GABA能突触,而这种突触只出现在轴突起始节段(AIS)。在这里,通过减少酪氨酸激酶受体ephrin A7(EphA7)的表达,我们选择性地损害了不同亚群中间神经元的调节功能,特别是针对主神经元的体节和近端树突。这种扰动引起了自发抑制性突触输入的预期减少和兴奋性自发突触活动的增加,这很可能是由于抑制张力的降低。此外,突触活动增加后,内在兴奋性也随之降低。BLA内的NF-KD会导致消减学习受损,但不会增加焦虑症状,而BLA内减少EphA7的表达会导致焦虑症状增加,这是在高架加迷宫中测得的结果,但不会影响恐惧条件反射或消减学习。这些结果证实了BLA和BLA内变态反应在应激诱导的焦虑症状加重和恐惧消退学习受损中的作用,但揭示了BLA内机制的不同。这些结果还证实了GABA能中间神经元对这些效应的贡献,但表明了BLA内中间神经元不同亚群的选择性作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Neurobiology of Stress
Biochemistry, Genetics and Molecular Biology-Biochemistry
CiteScore
9.40
自引率
4.00%
发文量
74
审稿时长
48 days
期刊介绍:
Neurobiology of Stress is a multidisciplinary journal for the publication of original research and review articles on basic, translational and clinical research into stress and related disorders. It will focus on the impact of stress on the brain from cellular to behavioral functions and stress-related neuropsychiatric disorders (such as depression, trauma and anxiety). The translation of basic research findings into real-world applications will be a key aim of the journal.
Basic, translational and clinical research on the following topics as they relate to stress will be covered:
Molecular substrates and cell signaling,
Genetics and epigenetics,
Stress circuitry,
Structural and physiological plasticity,
Developmental Aspects,
Laboratory models of stress,
Neuroinflammation and pathology,
Memory and Cognition,
Motivational Processes,
Fear and Anxiety,
Stress-related neuropsychiatric disorders (including depression, PTSD, substance abuse),
Neuropsychopharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


