Spectroscopic characterization of triazine based covalent organic framework tempted changes in the structure of hemoglobin
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-10-22
DOI:10.1016/j.saa.2024.125320
引用次数: 0
Abstract
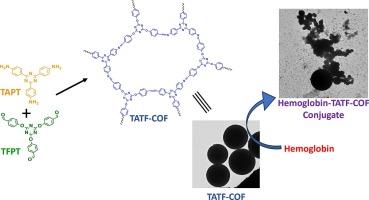
The present study aims to understand changes in the Hemoglobin (Hb) structure in the presence of a triazine based covalent organic framework (COF) through spectroscopic characterization. Covalent Organic Frameworks (COFs) due to their unique properties have been utilized in diverse fields including bio-applications. Utilization of COFs for conjugate formation with proteins will lead to the integration of biology and framework materials that can help in the development of bioconjugates for advanced bio-based applications such as diagnostics, therapeutics, and bioengineering. However, vital is to have a fundamental understanding of protein conformation in protein-COF conjugate. Herein, a triazine based COF has been synthesized via solvothermal method, termed TATF-COF which has been utilized for the formation of a conjugate with hemoglobin (Hb). Thereafter, studies have been performed to understand Hb structure in the presence of TATF-COF. Results from UV–vis, Fluorescence, and UV-CD spectroscopy studies revealed that in the presence of TATF-COF, there was a slight alteration in the Hb structure due to binding interactions between them and conjugate formation. Moreover, micrographs obtained from electron microscopy displayed formation of conjugate between Hb and TATF-COF result of binding interactions. DLS and zeta potential results also revealed conjugate formation due to binding interactions between TATF-COF and Hb. Thermal stability of Hb was also maintained as TATF-COF had insignificant effect on the Tm value of Hb. Overall, there was a slight alternation in the Hb native conformation due to binding interactions, however, TATF-COF was compatible with Hb as the protein’s native structure was well-preserved.
基于三嗪的共价有机框架的光谱特性诱导血红蛋白结构的变化
本研究旨在通过光谱特性分析,了解三嗪类共价有机框架(COF)存在时血红蛋白(Hb)结构的变化。共价有机框架(COF)因其独特的性质已被用于包括生物应用在内的多个领域。利用共价有机框架与蛋白质形成共轭物,将实现生物学与框架材料的整合,有助于开发生物共轭物,用于诊断、治疗和生物工程等先进的生物应用领域。然而,关键是要从根本上了解蛋白质-COF 共轭物中的蛋白质构象。在此,我们通过溶热法合成了一种三嗪基 COF,称为 TATF-COF,并将其用于与血红蛋白(Hb)形成共轭物。此后,研究人员对 TATF-COF 存在下的 Hb 结构进行了了解。紫外-可见光谱、荧光光谱和紫外-CD 光谱研究的结果表明,在 TATF-COF 的存在下,由于它们之间的结合作用和共轭物的形成,Hb 的结构发生了轻微的变化。此外,从电子显微镜获得的显微照片显示,Hb 和 TATF-COF 因结合相互作用而形成了共轭物。DLS 和 zeta 电位结果也显示,TATF-COF 与 Hb 之间的结合相互作用导致共轭物的形成。由于 TATF-COF 对 Hb 的 Tm 值影响不大,因此 Hb 的热稳定性也得以保持。总的来说,由于结合相互作用,Hb 的原生构象发生了轻微的变化,但是 TATF-COF 与 Hb 是兼容的,因为蛋白质的原生结构得到了很好的保留。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


