Heterodimeric studies of β-galactosidase genes as biocatalyst of lactose from Lactobacillus acidophilus MR-24
IF 4
Q2 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
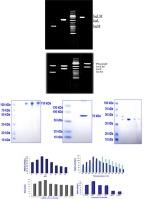
The current study characterized β-galactosidase-producing bacteria isolated from dairy products, in Lahore, Pakistan. Biochemical tests and the 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal) hydrolysis test identified 50 Lactobacillus isolates. Fifteen isolates with blue color on X-gal plates, L. acidophilus MR-24 displayed the highest enzyme activity (905.15 U/l), characterized based on 16S rRNA gene sequencing. The enzymatic activity was enhanced 10-fold by using a Sephadex G-75 column. The highest enzyme production was obtained at pH 7.0 and a temperature of 37 °C. The β-galactosidase (lac M, L, and LM with molecular weight 951 bp, 1887 bp, and 2.8 kb respectively), was extracted from L. acidophilus MR-24. It was ligated to the pTZ57R cloning vector after Polymerase chain reaction (PCR) and agarose gel analysis. The confirmation of cloning was done via colony PCR and restriction digestion analysis. Sequencing data indicated that the enzyme consists of two overlapping regions; lac L and M encoding 70 kDa and 35 kDa protein respectively. The β-galactosidase shows significant homology with the gene from other Lactobacillus sp. Compared to crude enzymes, the co-expression of lac L and M in E. coli produced active proteins with a 30-fold increase in activity after purification via ion-exchange chromatography. The purified enzyme revealed its maximal activity at pH 7 while pH 3.0 and 9.0 showed minimal activity. The optimum temperature was 60 °C for native and 45 °C for expressed enzyme, remaining 40 % activity at 90 °C. The enzyme’s 3-dimensional (3D) structure showed Domain N, A, and C. The L. acidophilus MR-24 strain, as a probiotic in dairy products, can provide benefits to individuals with lactose intolerance.
嗜酸乳杆菌 MR-24 中作为乳糖生物催化剂的 β-半乳糖苷酶基因的异构研究
本研究对从巴基斯坦拉合尔乳制品中分离出的产β-半乳糖苷酶细菌进行了鉴定。生化测试和 5-溴-4-氯-3-吲哚基-β-D-半乳糖苷(X-gal)水解测试确定了 50 个乳酸杆菌分离物。根据 16S rRNA 基因测序,15 个分离株在 X-gal 平板上呈蓝色,其中嗜酸乳杆菌 MR-24 的酶活性最高(905.15 U/l )。通过使用 Sephadex G-75 色谱柱,酶活性提高了 10 倍。在 pH 值为 7.0、温度为 37 ℃ 的条件下,酶的产量最高。从嗜酸乳杆菌 MR-24 中提取出了β-半乳糖苷酶(lac M、L 和 LM,分子量分别为 951 bp、1887 bp 和 2.8 kb)。经过聚合酶链式反应(PCR)和琼脂糖凝胶分析,将其连接到 pTZ57R 克隆载体上。通过菌落 PCR 和限制性消化分析确认了克隆。测序数据表明,该酶由两个重叠区域组成;Lac L 和 M 分别编码 70 kDa 和 35 kDa 蛋白。与粗酶相比,在大肠杆菌中共同表达的 lac L 和 M 产生的活性蛋白经离子交换色谱纯化后,其活性提高了 30 倍。纯化后的酶在 pH 值为 7 时活性最高,而 pH 值为 3.0 和 9.0 时活性最低。原生酶的最适温度为 60 ℃,表达酶的最适温度为 45 ℃,在 90 ℃ 时仍有 40% 的活性。嗜酸乳杆菌 MR-24 菌株作为乳制品中的益生菌,可为乳糖不耐症患者带来益处。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Biotechnology
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
6.70
自引率
3.60%
发文量
50
审稿时长
38 days
期刊介绍:
Current Research in Biotechnology (CRBIOT) is a new primary research, gold open access journal from Elsevier. CRBIOT publishes original papers, reviews, and short communications (including viewpoints and perspectives) resulting from research in biotechnology and biotech-associated disciplines.
Current Research in Biotechnology is a peer-reviewed gold open access (OA) journal and upon acceptance all articles are permanently and freely available. It is a companion to the highly regarded review journal Current Opinion in Biotechnology (2018 CiteScore 8.450) and is part of the Current Opinion and Research (CO+RE) suite of journals. All CO+RE journals leverage the Current Opinion legacy-of editorial excellence, high-impact, and global reach-to ensure they are a widely read resource that is integral to scientists' workflow.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


