Electrochemistry-enabled Rh-catalyzed regioselective [4 + 1] and [4 + 2] cycloaddition of benzoic acid with alkynyl esters/amides†‡
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A Rh(III)-catalyzed additive-controlled regioselective [4 + 1] and [4 + 2] cyclization of benzoic acids with alkynyl esters/amides under electrochemical conditions has been developed for the synthesis of phthalides and isocoumarins. The selectivity for the product formation was attained by merely changing the additive. Employment of NaOAc in the reaction produces isocoumarins whereas AcOH favors phthalides. Cyclic voltammetry and control experiments reveal the mechanistic pathway of the reaction.
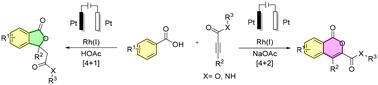
电化学催化的 Rh 催化苯甲酸与炔基酯/酰胺的区域选择性 [4 + 1] 和 [4 + 2] 环加成反应†‡
在电化学条件下,开发了一种 Rh(III) 催化的添加剂控制的苯甲酸与炔酯/酰胺的区域选择性 [4 + 1] 和 [4 + 2] 环化反应,用于合成邻苯二甲酸酯和异香豆素。只需改变添加剂,就能获得生成物的选择性。在反应中使用 NaOAc 可以生成异香豆素,而 AcOH 则有利于生成邻苯二甲酸酯。循环伏安法和对照实验揭示了反应的机理途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


