Evaluation on the recovery of lignin from basic [Ch][Lys] systems using low-cost alcohols as anti-solvents under acid-free conditions†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
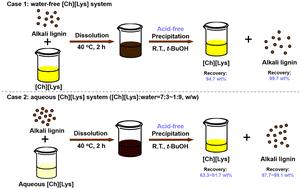
Delignification of lignocellulose using basic ionic liquids (BILs) such as choline lysinate ([Ch][Lys]) is a promising method due to its high efficiency, mild conditions, and low toxicity. Typically, the following precipitation of lignin by adding acid media makes it challenging to recycle BILs. Herein, we explored a series of low-cost and recyclable alcohols as anti-solvents, including methanol (MeOH), ethanol (EtOH), iso-propanol (i-PrOH), and tert-butanol (t-BuOH), for recovering [Ch][Lys] and precipitating lignin without adding an acid from water-free [Ch][Lys] (case 1) and aqueous [Ch][Lys] (case 2). For case 1, lignin recovery followed the order of EtOH > i-PrOH > t-BuOH (MeOH was not able to recover lignin and [Ch][Lys]), which was negatively correlated with their pKa values, indicating the effect of the inhibited generation of a basic anion (e.g. EtO− from EtOH) from –NH2 in [Ch][Lys] on lignin precipitation. t-BuOH showed the highest lignin recovery of 99.7%, ensuring the high purity of the recovered [Ch][Lys] (recovery of 94.7%). Lignin deprotonation and depolymerization were detected. For case 2, t-BuOH also facilitated the recovery of lignin from an aqueous lignin–[Ch][Lys] system with a nearly quantitative lignin recovery, yet with lower [Ch][Lys] recovery of 81.7% and 64.0% at the [Ch][Lys] : water ratios (w/w) of 7 : 3 and 1 : 9, respectively. The lower recovery of [Ch][Lys] might be due to the poor dispersity of lignin solid in t-BuOH, and water also enhanced the deprotonation of lignin, thus making lignin precipitation more difficult. Based on the results, a deprotonation-based lignin dissolution mechanism has been proposed, which also helps to understand lignin dissolution and precipitation in a [Ch][Lys]-based system.
在无酸条件下使用低成本醇类作为抗溶剂从碱性[Ch][Lys]体系中回收木质素的评估†。
使用碱性离子液体(BILs)(如赖氨酸胆碱([Ch][Lys]))对木质纤维素进行木质素化是一种很有前景的方法,因为它效率高、条件温和且毒性低。通常情况下,加入酸性介质后木质素会随之沉淀,这给 BILs 的回收利用带来了挑战。在此,我们探索了一系列低成本、可回收的醇类作为反溶剂,包括甲醇(MeOH)、乙醇(EtOH)、异丙醇(i-PrOH)和叔丁醇(t-BuOH),用于从无水[Ch][Lys](情况 1)和水性[Ch][Lys](情况 2)中回收[Ch][Lys]和沉淀木质素而无需加酸。在情况 1 中,木质素的回收顺序为 EtOH > i-PrOH > t-BuOH(MeOH 无法回收木质素和 [Ch][Lys]),这与它们的 pKa 值呈负相关,表明碱性阴离子(如 EtOH 中的 EtO-)的生成受到了抑制。t-BuOH的木质素回收率最高,达到 99.7%,确保了回收的[Ch][Lys]的高纯度(回收率为 94.7%)。检测到了木质素的去质子化和解聚。对于情况 2,t-BuOH 也有助于从木质素-[Ch][Lys]水溶液体系中回收木质素,其木质素回收率接近定量,但[Ch][Lys]回收率较低,[Ch][Lys]与水的比例(w/w)分别为 7 :3 和 1 : 9 时,[Ch][Lys]回收率分别为 81.7% 和 64.0%。Ch][Lys] 的回收率较低可能是由于木质素固体在 t-BuOH 中的分散性较差,而且水也会增强木质素的去质子化作用,从而使木质素沉淀更加困难。根据研究结果,提出了基于去质子化作用的木质素溶解机理,这也有助于理解[Ch][Lys]基体系中木质素的溶解和沉淀。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


