Light-induced isomerization of quinoline-N-oxide derivatives through Zn-catalysis: a photochemical approach for synthesizing 2-quinolinone derivatives†
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A novel strategy for the synthesis of quinolin-2(1H)-one and its related derivatives is described. The light-induced Zn-catalyzed isomerization of quinoline-N-oxides proceeds smoothly to afford 2-quinolinones in satisfactory to high yields. Control experiments and computational studies demonstrate that this photochemical isomerization reaction proceeds through an intramolecular hydrogen and oxygen transfer reaction, ensuring 100% atomic economy.
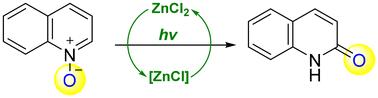
通过锌催化实现喹啉-N-氧化物衍生物的光诱导异构化:合成 2-喹啉酮衍生物的光化学方法†。
本文介绍了一种合成喹啉-2(1H)-酮及其相关衍生物的新策略。光诱导 Zn 催化的喹啉-N-氧化物异构化反应进展顺利,能以令人满意的高产率得到 2-喹啉酮。对照实验和计算研究表明,这种光化学异构化反应是通过分子内氢和氧转移反应进行的,确保了 100% 的原子经济性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


