Electroreductive deoxygenative carboxylation of alkyl oxalates with CO2†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
An electroreductive deoxygenative carboxylation of alkyl oxalates with CO2 is demonstrated for the first time, which offers a general and scalable method for obtaining various carboxylic acids. This electrochemical method features a broad substrate scope and mild reaction conditions; various secondary benzyl alcohols and non-benzylic alcohols, primary benzyl alcohols and non-benzylic alcohols, and tertiary benzyl alcohols and non-benzylic alcohols can be converted into the corresponding carboxylic acids in good to excellent yields. Importantly, this electroreductive deoxygenative carboxylation reaction also offers a mild and straightforward route to access drug molecules, such as ibuprofen, (±) naproxen, fenoprofen, hexaprofen, and biprofen.
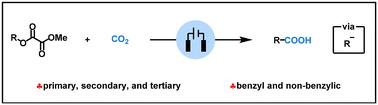
烷基草酸盐与 CO2† 的电还原脱氧羧化反应
首次展示了烷基草酸盐与二氧化碳的电还原脱氧羧化反应,为获得各种羧酸提供了一种通用的、可扩展的方法。这种电化学方法具有底物范围广、反应条件温和的特点;各种仲苄基醇和非苄基醇、伯苄基醇和非苄基醇以及叔苄基醇和非苄基醇都能以良好到极佳的产率转化为相应的羧酸。重要的是,这种电还原脱氧羧化反应还为布洛芬、(±)萘普生、非诺洛芬、己洛芬和联洛芬等药物分子的获取提供了一条温和而直接的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


