What to do with polyurethane waste? The environmental potential of chemically recycling polyurethane rigid foam†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Chemical recycling of plastics has gathered momentum to manage plastic waste and replace fossil-based feedstocks. However, chemical recycling of complex polymers, such as polyurethane (PU) rigid foam, can yield a variety of intermediates. But which intermediates reduce environmental impacts the most when produced from PU is currently unclear. In this work, we assess the potential of chemical recycling of PU rigid foam waste to reduce environmental impacts compared to state-of-the-art treatment options, such as incineration and landfilling. For this purpose, we extend the environmental potential methodology to account for any possible recycling product. We then calculate the environmental potential for six ideal closed-loop recycling options and one experimentally demonstrated recycling option based on a patent. All analyzed chemical recycling options for PU rigid foam to various intermediates are shown to offer a substantial environmental potential to reduce multiple environmental impacts. The best performing option recovers both polyol and isocyanate and can decrease climate change impacts by 3.8–5.6 kgCO2eq. per kg PU treated. However, PU rigid foam recycling to low-value intermediates, such as benzene, does not seem promising due to burden shifting actually increasing half of the analyzed environmental impacts. We further determine the minimal conversion rates required to reduce environmental impacts by chemical recycling of PU rigid foam. Our environmental potential analysis assists the decision-making process for product prioritization in recycling and identifies (side-)products whose recovery is worth investigating from an environmental perspective.
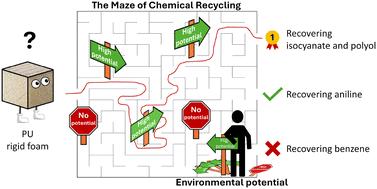
如何处理聚氨酯废料?化学回收聚氨酯硬质泡沫的环保潜力†...
塑料的化学回收利用在管理塑料废弃物和替代化石原料方面势头强劲。然而,对聚氨酯(PU)硬质泡沫塑料等复杂聚合物进行化学回收可产生多种中间体。但目前还不清楚用聚氨酯生产哪种中间体最能减少对环境的影响。在这项工作中,我们评估了与焚烧和填埋等最先进的处理方案相比,聚氨酯硬质泡沫废料的化学回收利用在减少环境影响方面的潜力。为此,我们扩展了环境潜力方法,以考虑任何可能的回收产品。然后,我们计算了六种理想的闭环回收方案和一种基于专利的实验验证回收方案的环境潜力。结果表明,所有经过分析的将聚氨酯硬质泡沫转化为各种中间体的化学回收方案都具有巨大的环境潜力,可减少多种环境影响。性能最好的方案可回收多元醇和异氰酸酯,每处理一千克聚氨酯可减少 3.8-5.6 千克二氧化碳当量的气候变化影响。然而,将聚氨酯硬质泡沫回收利用为苯等低价值中间体似乎并不乐观,因为负担转移实际上增加了一半的分析环境影响。我们进一步确定了聚氨酯硬质泡沫化学回收所需的最低转化率,以减少对环境的影响。我们的环境潜力分析有助于确定回收产品优先次序的决策过程,并从环境角度确定值得研究回收的(副)产品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


