Combined Phase 1/2a Initial Clinical Safety Trials and Proof-of-Concept Assessment of a Novel Antimicrobial Peptide KSL-W Anti-Plaque Chewing Gum
Abstract
Objectives
The effective control of dental plaque is crucial for oral health, given that pathogenic bacteria in plaque are the primary cause of dental caries. Current antimicrobial agents, although effective, disrupt the oral microbiome and lead to oral dysbiosis, hindering efforts to curb dental caries. Novel antimicrobial peptides offer a promising solution due to their selective bactericidal activity against cariogenic bacteria. This study explores the initial safety and efficacy of KSL-W formulated into chewing gum through a Phase 1 and 2a clinical trial.
Methods
The combined trial, approved by the FDA, follows a double-blind, randomized, placebo-controlled design. Phase 1 assessed safety with single doses (2−100 mg), whereas Phase 2a explored both safety and proof of concept in reducing oral bacteria with multiple doses (4−75 mg). Besides adverse events (Phase 1), outcome measures included whole-mouth plaque and gingival index scores and bleeding on probing (Phase 2a).
Results
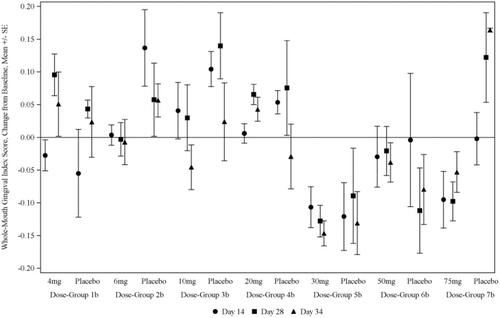
KSL-W demonstrated safety in both phases, with no severe adverse events. The proof-of-concept analysis revealed a decrease in plaque and gingival inflammation, particularly at doses ≥ 20 mg. The 30 mg dose appeared to yield optimal effects without any adverse reactions in subjects.
Conclusions
Results from this study indicate that KSL-W is safe for use in humans and provides initial evidence of its potential efficacy in reducing plaque and gingival inflammation. Further research is essential to determine optimal usage and ultimate safety, and to assess its potential in diverse populations.
Trial Registration
The trial is registered with the FDA (Trial Registration Number: NCT01877421). The clinical trials were registered in the clinicaltrials.gov database under the title “Safety and Tolerability of Antiplaque Chewing Gum in a Gingivitis Population” and the identifier number is NCT01877421. The URL for accessing the study in clinicaltrials.gov is https://clinicaltrials.gov/study/NCT01877421?intr=Antiplaque%20chewing&rank=1.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


