Natural phenol carbamates: Selective BuChE/FAAH dual inhibitors show neuroprotection in an Alzheimer's disease mouse model
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
FAAH inhibition can indirectly enhance endocannabinoid signaling to therapeutic levels, effectively preventing or slowing its progression of Alzheimer's disease (AD). Hence, the search for effective dual FAAH/cholinesterase inhibitors is considerable need for disease-modifying therapies. To this aim, we designed, synthesized, and tested three series of natural phenol carbamates. The majority of carbamates proved to be potent on a single target, amongst them, compound D12 containing paeonol motif was identified as an effective dual BuChE/FAAH inhibitor, with well-balanced nanomolar activity (IC50 = 81 and 400 nM for hBuChE and hFFAH, respectively). D12 possessed BBB penetrating ability, benign safety, neuroprotection and pseudo-irreversible BuChE inhibition (Kd = 2.11 μM, k2 = 2.27 min−1), showing good drug-like properties. D12 also modulated the BV2 microglial polarization to inhibit neuroinflammation. In vivo study verified that D12 improved Aβ1-41-induced learning impairments in AD mouse model for both short- and long-term memory responses. Thus, the dual activity of D12 could lead to a potentially more effective treatment for the counteraction of AD progression.
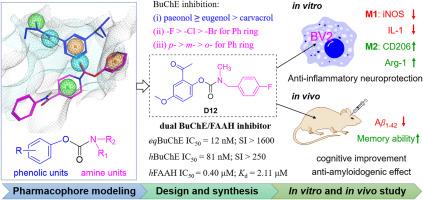
天然苯酚氨基甲酸酯:选择性 BuChE/FAAH 双抑制剂在阿尔茨海默病小鼠模型中显示出神经保护作用
FAAH 抑制可间接增强内源性大麻素信号转导至治疗水平,从而有效预防或减缓阿尔茨海默病(AD)的进展。因此,寻找有效的 FAAH/胆碱酯酶双重抑制剂是改变疾病疗法的巨大需求。为此,我们设计、合成并测试了三个系列的天然苯酚氨基甲酸酯。其中,含有芍药酚基团的化合物 D12 被鉴定为一种有效的 BuChE/FAAH 双重抑制剂,具有均衡的纳摩尔活性(对 hBuChE 和 hFFAH 的 IC50 分别为 81 和 400 nM)。D12具有BBB穿透能力、良性安全性、神经保护作用和假不可逆的BuChE抑制作用(Kd = 2.11 μM,k2 = 2.27 min-1),显示出良好的类药物特性。D12 还能调节 BV2 小胶质细胞的极化,从而抑制神经炎症。体内研究证实,D12能改善Aβ1-41诱导的AD小鼠学习障碍,包括短期和长期记忆反应。因此,D12的双重活性可能会导致一种更有效的治疗方法,以对抗AD的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


