Recommendations to promote equity, diversity and inclusion in decentralized clinical trials
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
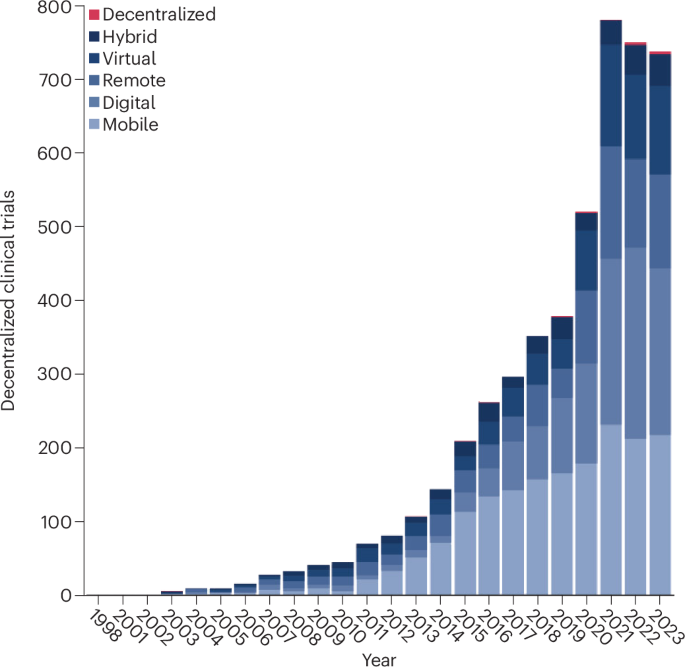
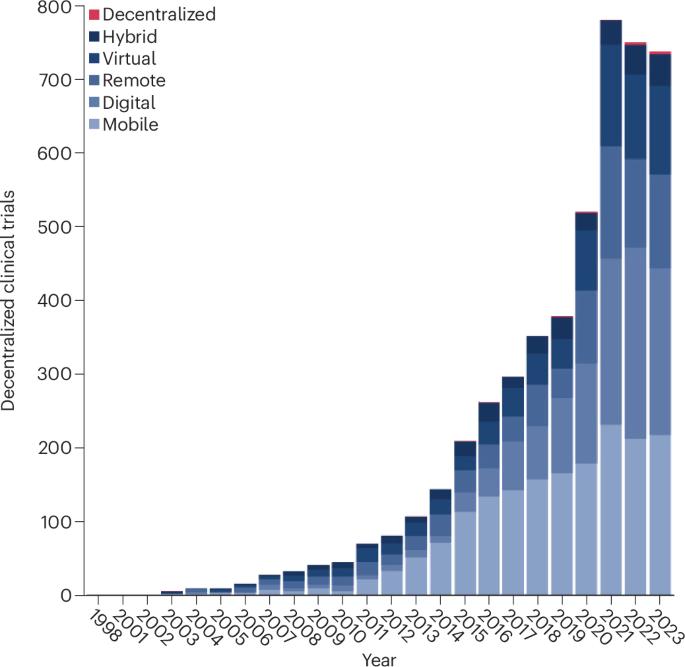
Decentralized clinical trials involving the use of digital tools to facilitate remote research are gaining momentum. Rapid advancements in digital technologies have supported the adoption of these trials. These innovations facilitate virtual interactions between clinical trial teams and participants by making it easier to collect, transfer and store electronic data. While some studies have demonstrated the potential for these approaches to reduce barriers to clinical trial participation, they are associated with several challenges that may create or worsen existing health inequalities and limit the generalizability of trial results. Here we review the potential for digitally enabled and decentralized clinical trials to enhance clinical trial participation in an equitable manner. We describe the key barriers individuals from underserved groups may face, and provide recommendations to promote equity, diversity and inclusion. Digitally enabled and decentralized clinical trials could enable large-scale recruitment of diverse participants—but careful consideration of the barriers faced by underserved groups will be crucial to their success.
促进分散临床试验中的公平、多样性和包容性的建议
使用数字工具促进远程研究的分散式临床试验的发展势头日益强劲。数字技术的快速发展为这些试验的采用提供了支持。这些创新技术通过简化电子数据的收集、传输和存储,促进了临床试验团队与参与者之间的虚拟互动。虽然一些研究已经证明了这些方法在减少临床试验参与障碍方面的潜力,但它们也带来了一些挑战,可能会造成或加剧现有的健康不平等,并限制试验结果的普遍性。在此,我们回顾了数字化和分散化临床试验以公平方式提高临床试验参与度的潜力。我们描述了来自服务不足群体的个人可能面临的主要障碍,并提出了促进公平、多样性和包容性的建议。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


