Attenuated Natural Convection in Molten Salt Electrochemical Systems with Minimal Heat Dissipation
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The accurate understanding of mass transfer in molten salt contributes to revealing the reaction mechanism and advancing the technologies. The existence of notable natural convection effects has been demonstrated in our previous studies, even though the driving forces for such a high natural convection are still not clear. Herein, we showed that the intense natural convection in molten salts resulted from severe heat dissipation through the electrodes (or the system). With an adiabatic design, natural convection effects were significantly suppressed in molten LiCl–KCl. The derived value of the natural convection layer (δconv) ranged from 190 to 250 μm in molten LiCl–KCl containing a redox couple (e.g., SmCl3, EuCl3, and CrCl3), comparable to those in aqueous solutions. The values of δconv in LiCl–KCl–173 mM CrCl3 increased to ∼360 μm due to the change in salt viscosity. The density-driven convection became dominant under a high redox concentration, and the increasing working temperature had no apparent effect on the natural convection effects because of the adiabatic design.
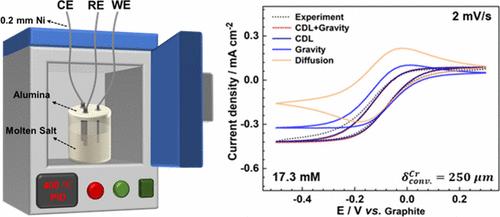
最小散热量的熔盐电化学系统中的衰减自然对流
准确理解熔盐中的传质有助于揭示反应机理和推动技术进步。我们之前的研究已经证明存在显著的自然对流效应,但如此高的自然对流的驱动力仍不明确。在这里,我们证明了熔盐中强烈的自然对流是由于电极(或系统)严重散热造成的。通过绝热设计,熔融锂盐-氯化钾中的自然对流效应被显著抑制。在含有氧化还原对偶(如 SmCl3、EuCl3 和 CrCl3)的熔融氯化锂-氯化钾中,自然对流层(δconv)的推导值介于 190 至 250 μm 之间,与水溶液中的自然对流层值相当。由于盐粘度的变化,LiCl-KCl-173 mM CrCl3 中的δconv 值增至 ∼360 μm。在高氧化还原浓度下,密度驱动对流成为主导,由于采用绝热设计,工作温度的升高对自然对流效应没有明显影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


