Dynamic sampling of a surveillance state enables DNA proofreading by Cas9
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
CRISPR-Cas9 has revolutionized genome engineering applications by programming its single-guide RNA, where high specificity is required. However, the precise molecular mechanism underscoring discrimination between on/off-target DNA sequences, relative to the guide RNA template, remains elusive. Here, using methyl-based NMR to study multiple holoenzymes assembled in vitro, we elucidate a discrete protein conformational state which enables recognition of DNA mismatches at the protospacer adjacent motif (PAM)-distal end. Our results delineate an allosteric pathway connecting a dynamic conformational switch at the REC3 domain, with the sampling of a catalytically competent state by the HNH domain. Our NMR data show that HiFi Cas9 (R691A) increases the fidelity of DNA recognition by stabilizing this "surveillance state" for mismatched substrates, shifting the Cas9 conformational equilibrium away from the active state. These results establish a paradigm of substrate recognition through an allosteric protein-based switch, providing unique insights into the molecular mechanism which governs Cas9 selectivity.
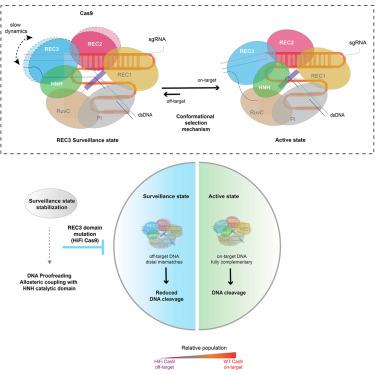

监控状态的动态取样使 Cas9 能够进行 DNA 校对
CRISPR-Cas9 通过对需要高特异性的单导 RNA 进行编程,彻底改变了基因组工程应用。然而,相对于引导 RNA 模板而言,区分目标 DNA 序列的精确分子机制仍未确定。在这里,我们利用基于甲基的核磁共振技术研究了体外组装的多个全酶,阐明了一种离散的蛋白质构象状态,它能识别原间隔邻接基序(PAM)远端的 DNA 错配。我们的研究结果勾勒出了一条异构途径,它将 REC3 结构域的动态构象转换与 HNH 结构域的催化状态取样连接起来。我们的核磁共振数据显示,HiFi Cas9 (R691A)通过稳定这种针对不匹配底物的 "监视状态",使 Cas9 的构象平衡偏离活性状态,从而提高了 DNA 识别的保真度。这些结果建立了一种通过基于异构蛋白的开关来识别底物的范例,为研究支配 Cas9 选择性的分子机制提供了独特的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


