The CRISPR-associated adenosine deaminase Cad1 converts ATP to ITP to provide antiviral immunity
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Type III CRISPR systems provide immunity against genetic invaders through the production of cyclic oligo-adenylate (cAn) molecules that activate effector proteins that contain CRISPR-associated Rossman fold (CARF) domains. Here, we characterized the function and structure of an effector in which the CARF domain is fused to an adenosine deaminase domain, CRISPR-associated adenosine deaminase 1 (Cad1). We show that upon binding of cA4 or cA6 to its CARF domain, Cad1 converts ATP to ITP, both in vivo and in vitro. Cryoelectron microscopy (cryo-EM) structural studies on full-length Cad1 reveal an hexameric assembly composed of a trimer of dimers, with bound ATP at inter-domain sites required for activity and ATP/ITP within deaminase active sites. Upon synthesis of cAn during phage infection, Cad1 activation leads to a growth arrest of the host that prevents viral propagation. Our findings reveal that CRISPR-Cas systems employ a wide range of molecular mechanisms beyond nucleic acid degradation to provide adaptive immunity in prokaryotes.
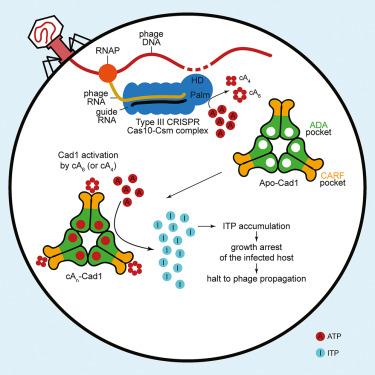
CRISPR相关腺苷脱氨酶Cad1将ATP转化为ITP,提供抗病毒免疫力
III型CRISPR系统通过产生环状低聚腺苷酸(cAn)分子来激活含有CRISPR相关罗斯曼折叠(CARF)结构域的效应蛋白,从而提供抵御基因入侵者的免疫力。在这里,我们描述了一种效应蛋白的功能和结构,这种效应蛋白的CARF结构域与腺苷脱氨酶结构域融合,即CRISPR相关腺苷脱氨酶1(Cad1)。我们发现,当 cA4 或 cA6 与其 CARF 结构域结合后,Cad1 在体内和体外都能将 ATP 转化为 ITP。对全长 Cad1 的冷冻电子显微镜(cryo-EM)结构研究显示,Cad1 是由三聚体和二聚体组成的六聚体,结合的 ATP 位于活性所需的结构域间位点,ATP/ITP 位于脱氨酶活性位点。在噬菌体感染过程中合成 cAn 时,Cad1 激活会导致宿主生长停滞,从而阻止病毒传播。我们的研究结果表明,CRISPR-Cas系统在核酸降解之外还采用了多种分子机制,为原核生物提供适应性免疫。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


