Hexokinase 2 senses fructose in tumor-associated macrophages to promote colorectal cancer growth
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Fructose is associated with colorectal cancer tumorigenesis and metastasis through ketohexokinase-mediated metabolism in the colorectal epithelium, yet its role in the tumor immune microenvironment remains largely unknown. Here, we show that a modest amount of fructose, without affecting obesity and associated complications, promotes colorectal cancer tumorigenesis and growth by suppressing the polarization of M1-like macrophages. Fructose inhibits M1-like macrophage polarization independently of fructose-mediated metabolism. Instead, it serves as a signal molecule to promote the interaction between hexokinase 2 and inositol 1,4,5-trisphophate receptor type 3, the predominant Ca2+ channel on the endoplasmic reticulum. The interaction reduces Ca2+ levels in cytosol and mitochondria, thereby suppressing the activation of mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 1 (STAT1) as well as NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome activation. Consequently, this impedes M1-like macrophage polarization. Our study highlights the critical role of fructose as a signaling molecule that impairs the polarization of M1-like macrophages for tumor growth.
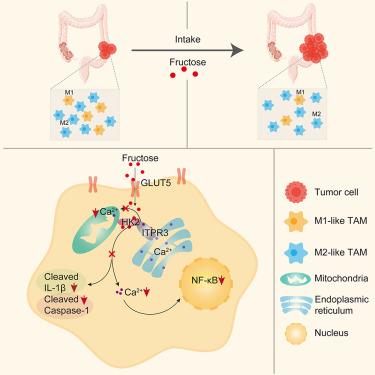
己糖激酶 2 能感知肿瘤相关巨噬细胞中的果糖,从而促进结直肠癌的生长
果糖通过酮六磷酸酶介导的结直肠上皮代谢与结直肠癌肿瘤发生和转移有关,但它在肿瘤免疫微环境中的作用在很大程度上仍不为人所知。在这里,我们发现,在不影响肥胖和相关并发症的情况下,适量的果糖可通过抑制 M1 样巨噬细胞的极化来促进结直肠癌肿瘤的发生和生长。果糖抑制 M1 样巨噬细胞极化与果糖介导的新陈代谢无关。相反,果糖是一种信号分子,可促进己糖激酶 2 与内质网上主要的 Ca2+ 通道肌醇 1,4,5- 三磷酸受体 3 型之间的相互作用。这种相互作用降低了细胞质和线粒体中的 Ca2+ 水平,从而抑制了丝裂原活化蛋白激酶(MAPK)和转录信号转导和激活因子 1(STAT1)的激活,以及 NOD-、LRR- 和含吡咯啉结构域蛋白 3(NLRP3)炎性体的激活。因此,这阻碍了 M1 样巨噬细胞的极化。我们的研究强调了果糖作为一种信号分子的关键作用,它能阻碍 M1 样巨噬细胞极化以促进肿瘤生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


