Stereodivergent Synthesis of Atropisomeric Indole-Fused δ-Lactams Bearing All-Carbon Quaternary Stereocenters via Cu-Catalyzed Desymmetric Arene Amidation
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Great achievements have been made in constructing valuable chiral lactams via noble metal-catalyzed intramolecular γ-amidations using dioxazolones as nitrene precursors. However, most of them are limited to synthesizing central chirality, and the preparation of all-carbon quaternary stereocenters and C−N axial chirality is extremely challenging and still under-explored. Herein, a Cu-catalyzed desymmetric arene δ-lactamizations of dioxazolones is disclosed, leading to the diastereodivergent synthesis of chiral indole-fused δ-lactams bearing all-carbon quaternary stereocenters and C−N axial chirality in generally good yield, stereoselectivity, and regioselectivity with wide substrate scope. Interestingly, all four stereoisomers of δ-lactams containing all-carbon quaternary stereocenters and C−N axial chirality can be readily achieved simply by varying the configurations of the single chiral copper catalyst and base treatments. Additionally, this reaction probably undergoes a Cu-catalyzed singlet nitrene transfer/rearrangement and remote enantiocontrol process strongly supported by control experiments and theoretical calculations.
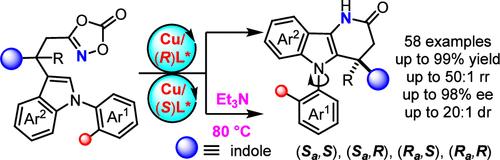
通过铜催化非对称烯丙基酰胺化反应合成具有全碳季立体中心的异构吲哚融合δ-内酰胺
以二噁唑酮为腈类前体,通过贵金属催化的分子内γ-酰胺化反应构建有价值的手性内酰胺方面取得了巨大成就。然而,这些研究大多局限于合成中心手性,而制备全碳四元立体中心和 C-N 轴向手性极具挑战性,目前仍处于探索阶段。本文公开了一种 Cu 催化的二恶唑酮非对称炔δ-内酰胺化方法,从而非对映地发散合成了具有全碳四元立体中心和 C-N 轴手性的手性吲哚融合δ-内酰胺,该方法具有普遍良好的收率、立体选择性和区域选择性,且具有广泛的底物范围。有趣的是,只需改变单一手性铜催化剂和碱处理的配置,就能轻松获得含有全碳四元立体中心和 C-N 轴手性的δ-内酰胺的所有四种立体异构体。此外,该反应可能经历了一个铜催化的单子腈转移/重排和远程对映控制过程,这一点得到了控制实验和理论计算的有力支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


