Kinetic analysis of the self-discharge of the NiOOH OER active phase in KOH electrolyte: insights from in-situ Raman and UV–Vis reflectance spectroscopies
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
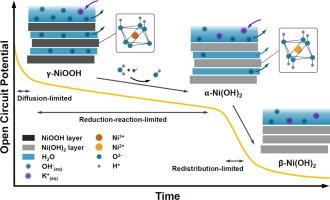
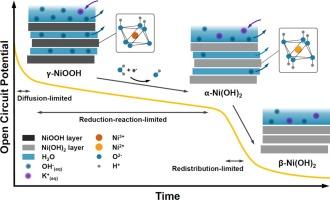
NiOOH has been established as the active phase of NiO-based electrocatalysts in the alkaline Oxygen Evolution Reaction (OER). Here, we investigate the self-discharge behavior of NiOOH electrodes under open circuit potential (OCP) conditions in 1 M KOH electrolyte by monitoring phase changes via in-situ Raman and UV–Vis reflectance spectroscopies and performing kinetic analyses on the OCP and spectroscopic data. Our findings reveal a linear phase change from NiOOH to Ni(OH)2 over time, indicative of a 0th-order reduction reaction. Contrarily, the OCP evolution associated with this phase reduction displayed a combination of linear and exponential decay patterns as a result of various kinetics, including Faradaic processes and diffusion-controlled mechanisms, influencing the self-discharge potential over 1.25 V (vs RHE). An additional linear region at lower potentials (<1.25 V (vs RHE)) suggests that charge redistribution due to the phase change from α-Ni(OH)2 to β-Ni(OH)2 dominates the self-discharge, a behavior confirmed by in-situ UV–Vis reflectance spectroscopy. These findings highlight the effectiveness of combining in-situ Raman and UV–Vis spectroscopy with electrochemical data for real-time monitoring of electrochemical processes, here potential-dependent electrocatalyst phase changes, leading to a more detailed and accurate understanding of the dynamic behavior, phase change kinetics, and self-discharge behaviors of solid electrocatalysts that can guide the design of more efficient and durable energy storage and conversion materials.
KOH 电解液中 NiOOH OER 活性相的自放电动力学分析:原位拉曼光谱和紫外可见反射光谱的启示
在碱性氧进化反应(OER)中,NiOOH 已被确定为基于 NiO 的电催化剂的活性相。在此,我们通过原位拉曼光谱和紫外可见反射光谱监测相变,并对 OCP 和光谱数据进行动力学分析,研究了 NiOOH 电极在 1 M KOH 电解液中开路电位 (OCP) 条件下的自放电行为。我们的研究结果表明,随着时间的推移,从 NiOOH 到 Ni(OH)2 的相变呈线性变化,这表明发生了 0 阶还原反应。与此相反,与这种相还原相关的 OCP 演变显示出线性和指数衰减模式的结合,这是各种动力学(包括法拉第过程和扩散控制机制)的结果,影响了 1.25 V 以上的自放电电位(相对于 RHE)。在较低电位(<1.25 V (vs RHE))下的额外线性区域表明,从 α-Ni(OH)2 到 β-Ni(OH) 2 的相变引起的电荷再分布主导了自放电,原位紫外可见反射光谱证实了这一行为。这些发现凸显了将原位拉曼光谱和紫外可见光谱与电化学数据相结合,实时监测电化学过程(这里指电势相关的电催化剂相变)的有效性,从而更详细、更准确地了解固体电催化剂的动态行为、相变动力学和自放电行为,为设计更高效、更耐用的储能和转换材料提供指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


