Halogenated solvation structure and preferred Zn (002) deposition via trace additive towards high reversibility for aqueous zinc-ion batteries
IF 18.9
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Tremendous progress has been achieved in cathode materials for aqueous zinc-ion batteries (AZIBs), however their practical applications are hindered by the poor cycling stability of Zn anode. Herein, amphiphilic choline bromine (ChBr) is introduced as additive into highly-concentrated ZnSO4 aqueous electrolyte, which not only regulates the traditional Zn2+ solvation structure but also establishes an electrostatic shielding layer at electrolyte-anode interface. Compared to the pristine 3 M ZnSO4 electrolyte, ChBr-modified ZnSO4 electrolyte (ZSO-ChBr) is proven effective in promoting the reversibility of zinc plating/stripping and the preferred growth of Zn (002) plane, as well as suppressing the hydrogen evolution and side reactions on Zn anode surface. As a result, Zn||Zn symmetric cell in optimal ZSO-ChBr electrolyte could harvest a remarkable lifespan over 6000 h at 5 mA cm−2 and 1 mAh cm−2, besides a highly-reversible zinc plating/stripping process over 1500 cycles was achieved in Zn||Cu asymmetric cell. Moreover, the Zn||MnO2 full cell could exhibit an excellent rate capability and the cycling stability with a capacity retention of 80 % after 400 cycles. This work provides an integrated strategy of electrolyte engineering to elevate the desolvation kinetics of Zn2+ at anode-electrolyte interface and enhance the cycling stability of Zn anode, effectively improving the electrochemical performances of aqueous zinc-ion batteries (AZIBs) and promoting the development of various energy storage systems.
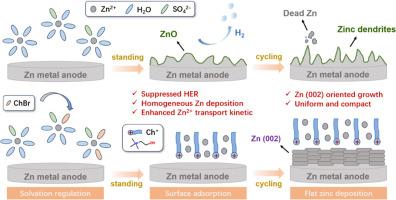
通过微量添加剂实现卤化溶解结构和优先锌 (002) 沉积,从而实现锌离子水电池的高可逆性
水性锌离子电池(AZIBs)的阴极材料取得了巨大进步,但由于锌阳极的循环稳定性较差,阻碍了其实际应用。在这里,两亲性胆碱溴(ChBr)作为添加剂被引入到高浓度的 ZnSO4 水电解液中,它不仅调节了传统的 Zn2+ 溶解结构,还在电解液-阳极界面上建立了静电屏蔽层。与原始的 3 M ZnSO4 电解质相比,ChBr 改性 ZnSO4 电解质(ZSO-ChBr)被证明能有效促进锌镀层/剥离的可逆性和 Zn (002) 平面的优先生长,并抑制锌阳极表面的氢演化和副反应。因此,在最佳的 ZSO-ChBr 电解液中,Zn||Zn 对称电池在 5 mA cm-2 和 1 mAh cm-2 的条件下可获得超过 6000 小时的超长寿命。此外,Zn||MnO2 全电池还表现出卓越的速率能力和循环稳定性,400 次循环后容量保持率达 80%。这项工作提供了一种电解质工程的综合策略,以提高阳极-电解质界面上Zn2+的解溶解动力学,并增强锌阳极的循环稳定性,从而有效改善水性锌离子电池(AZIBs)的电化学性能,促进各种储能系统的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


