Insight into formaldehyde decomposition over MOFs-derived CeO2-MnOx bimetallic oxides
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The ubiquitous presence of formaldehyde as a pollutant has aroused significant environmental and health concerns. The design and performance of (OR: transition metal oxide) catalysts in the catalytic oxidation method continue to face a myriad of challenges. Herein, a series of CeO2-x-MnOx catalysts are synthesized using manganous nitrate impregnated Ce-metal–organic-frameworks as the precursor, followed by a traditional calcination step. Interestingly, we found that the gases released during the pyrolysis of metal–organic-frameworks significantly affect the valence states of Ce and Mn, which are key factors responsible for catalytic activity. Characterizations results show that the CeO2-x-MnOx-2.5 sample contains a large amount of Ce3+, a high Mn3+/Mn4+ ratio, and an abundance of reactive oxygen species on its surface. Density functional theory results demonstrate that oxygen vacancies not only effectively suppress charge loss of Mn and Ce atoms but also significantly enhance the adsorption strength of CeO2-x-MnOx-2.5 for both formaldehyde and O2. These structural features jointly influence the adsorption as well as the rapid oxidation of formaldehyde molecules, leading to the excellent catalytic performance towards formaldehyde oxidation. This study provides a promising platform for designing straightforward, cost-effective, and highly efficient bimetallic catalysts suitable for low-temperature formaldehyde oxidation.
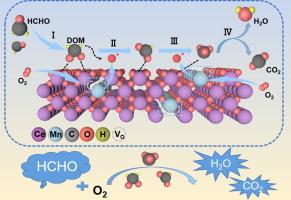
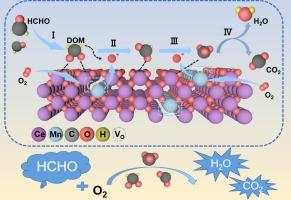
透视源自 MOFs 的 CeO2-MnOx 双金属氧化物分解甲醛的过程
甲醛作为一种污染物无处不在,引起了人们对环境和健康的极大关注。催化氧化法中过渡金属氧化物催化剂的设计和性能仍然面临着无数挑战。本文以硝酸锰浸渍的金属有机框架为前驱体,通过传统的煅烧步骤合成了一系列 CeO2-x-MnOx 催化剂。有趣的是,我们发现金属有机框架热解过程中释放出的气体会显著影响铈和锰的价态,而这正是催化活性的关键因素。表征结果表明,CeO2-x-MnOx-2.5 样品含有大量的 Ce3+,Mn3+/Mn4+ 比例较高,表面存在大量活性氧。密度泛函理论结果表明,氧空位不仅能有效抑制 Mn 原子和 Ce 原子的电荷损耗,还能显著增强 CeO2-x-MnOx-2.5 对甲醛和 O2 的吸附强度。这些结构特征共同影响了甲醛分子的吸附和快速氧化,从而实现了对甲醛氧化的优异催化性能。这项研究为设计适用于低温甲醛氧化的简单、经济、高效的双金属催化剂提供了一个前景广阔的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


