Simultaneous estimation of prasugrel and aspirin in bulk drugs by chemometric methods
IF 4.3
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-10-22
DOI:10.1016/j.saa.2024.125306
引用次数: 0
Abstract
A UV–Vis spectrophotometric method enhanced by chemometric techniques, specifically Principal Component Regression (PCR) and Partial Least Squares (PLS) regression, was developed and validated for the simultaneous quantification of prasugrel (PRA) and aspirin (ASP) in bulk drugs and pharmaceutical formulations. The method demonstrated high accuracy, precision, and robustness, achieving mean recoveries of 100.63% for PRA and 100.08% for ASP with relative standard deviations (RSD) below 3%. Both PCR and PLS models showed excellent predictive capabilities, with RMSEP values of 0.45–0.48 for PRA and 0.78–1.13 for ASP, indicating the models’ reliability. In line with green and white chemistry principles, the method minimizes environmental impact by reducing solvent consumption and waste generation compared to traditional chromatographic methods. The Analytical Eco-Scale score was 84, reflecting excellent compliance with green chemistry standards. The method’s simplicity, low energy consumption, and reduced chemical waste further support its alignment with sustainability goals. However, acetonitrile, a hazardous solvent, was still used in small quantities, and solvent recycling was not implemented, slightly affecting the eco-score. To evaluate the method’s greenness, the RGB12 algorithm was applied, achieving a high score of 94.4%, with the majority of parameters related to reagent consumption, waste production, energy efficiency, and safety scoring optimally. The method’s safety, cost-effectiveness, and minimal environmental footprint make it suitable for routine pharmaceutical analysis, particularly in quality control environments where resource efficiency and sustainability are prioritized. Thus, the developed method offers a sustainable, efficient, and environmentally friendly solution for the simultaneous analysis of prasugrel and aspirin in pharmaceutical formulations, making it a valuable tool for routine analysis in the pharmaceutical industry.
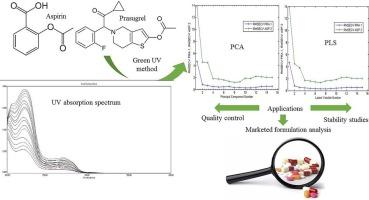
用化学计量法同时估算散装药物中的普拉格雷和阿司匹林。
采用化学计量学技术,特别是主成分回归(PCR)和偏最小二乘法(PLS),建立并验证了紫外可见分光光度法同时定量检测散装药物和药物制剂中的普拉格雷(PRA)和阿司匹林(ASP)。该方法准确度、精密度和稳健性都很高,PRA 的平均回收率为 100.63%,ASP 的平均回收率为 100.08%,相对标准偏差 (RSD) 低于 3%。PCR 和 PLS 模型都显示出卓越的预测能力,PRA 的 RMSEP 值为 0.45-0.48,ASP 的 RMSEP 值为 0.78-1.13,这表明模型是可靠的。与传统色谱法相比,该方法符合绿色和白色化学原则,减少了溶剂消耗和废物产生,从而最大限度地降低了对环境的影响。分析生态尺度得分为 84 分,非常符合绿色化学标准。该方法的简便性、低能耗和化学废物的减少进一步支持了其与可持续发展目标的一致性。不过,仍使用了少量乙腈(一种有害溶剂),而且没有实施溶剂回收,这对生态得分略有影响。为了评估该方法的绿色程度,采用了 RGB12 算法,获得了 94.4% 的高分,其中与试剂消耗、废物产生、能源效率和安全性相关的大部分参数都达到了最佳得分。该方法的安全性、成本效益和最小环境足迹使其适用于常规药物分析,特别是在优先考虑资源效率和可持续性的质量控制环境中。因此,所开发的方法为同时分析药物制剂中的普拉格雷和阿司匹林提供了一种可持续、高效和环保的解决方案,使其成为制药行业常规分析的重要工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


