A biodegradable lipid nanoparticle delivers a Cas9 ribonucleoprotein for efficient and safe in situ genome editing in melanoma
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The development of melanoma is closely related to Braf gene, which is a suitable target for CRISPR/Cas9 based gene therapy. CRISPR/Cas9-sgRNA ribonucleoprotein complexes (RNPs) stand out as the safest format compared to plasmid and mRNA delivery. Similarly, lipid nanoparticles (LNPs) emerge as a safer alternative to viral vectors for delivering the CRISPR/Cas9-sgRNA gene editing system. Herein, we have designed multifunctional cationic LNPs specifically tailored for the efficient delivery of Cas9 RNPs targeting the mouse Braf gene through transdermal delivery, aiming to treat mouse melanoma. LNPs are given a positive charge by the addition of a newly synthesized polymer, deoxycholic acid modified polyethyleneimine (PEI-DOCA). Positive charge enables LNPs to be delivered in vivo by binding to negatively charged cell membranes and proteins, thereby facilitating efficient skin penetration and enhancing the delivery of RNPs into melanoma cells for gene editing purposes. Our research demonstrates that these LNPs enhance drug penetration through the skin, successfully delivering the Cas9 RNPs system and specifically targeting the Braf gene. Cas9 RNPs loaded LNPs exert a notable impact on gene editing in melanoma cells, significantly suppressing their proliferation. Furthermore, in mice experiments, the LNPs exhibited skin penetration and tumor targeting capabilities. This innovative LNPs delivery system offers a promising gene therapy approach for melanoma treatment and provides fresh insights into the development of safe and effective delivery systems for Cas9 RNPs in vivo.
Statement of significance
CRISPR/Cas9 technology brings new hope for cancer treatment. Cas9 ribonucleoprotein offers direct genome editing, yet delivery challenges persist. For melanoma, transdermal delivery minimizes toxicity but faces skin barrier issues. We designed multifunctional lipid nanoparticles (LNPs) for Cas9 RNP delivery targeting the Braf gene. With metal microneedle pretreatment, our LNPs effectively edited melanoma cells, reducing Braf expression and inhibiting tumor growth. Our study demonstrates LNPs' potential for melanoma therapy and paves the way for efficient in vivo Cas9 RNP delivery systems in cancer therapy.
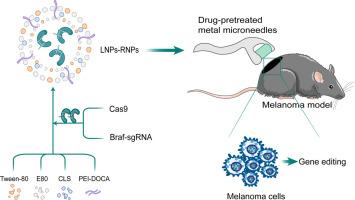
一种可生物降解的脂质纳米颗粒可输送 Cas9 核糖核蛋白,用于对黑色素瘤进行高效、安全的原位基因组编辑。
黑色素瘤的发生与 Braf 基因密切相关,而 Braf 基因是 CRISPR/Cas9 基因疗法的合适靶点。与质粒和 mRNA 相比,CRISPR/Cas9-sgRNA 核糖核蛋白复合物(RNPs)是最安全的递送形式。同样,脂质纳米颗粒(LNPs)作为病毒载体的一种更安全的替代品,可用于递送 CRISPR/Cas9-sgRNA 基因编辑系统。在此,我们设计了多功能阳离子 LNPs,专门用于通过透皮给药高效递送靶向小鼠 Braf 基因的 Cas9 RNPs,旨在治疗小鼠黑色素瘤。通过添加一种新合成的聚合物--脱氧胆酸改性聚乙烯亚胺(PEI-DOCA),LNPs 被赋予了正电荷。正电荷使 LNPs 能够通过与带负电荷的细胞膜和蛋白质结合而在体内递送,从而促进皮肤的有效渗透,并增强 RNPs 向黑色素瘤细胞的递送,以达到基因编辑的目的。我们的研究表明,这些 LNPs 可增强药物在皮肤中的穿透力,成功地递送 Cas9 RNPs 系统并特异性地靶向 Braf 基因。负载了 Cas9 RNPs 的 LNPs 对黑色素瘤细胞的基因编辑产生了显著影响,大大抑制了它们的增殖。此外,在小鼠实验中,LNPs 表现出皮肤穿透和肿瘤靶向能力。这种创新的 LNPs 运送系统为黑色素瘤治疗提供了一种前景广阔的基因治疗方法,并为开发安全有效的 Cas9 RNPs 体内运送系统提供了新的见解。意义说明:CRISPR/Cas9 技术为癌症治疗带来了新希望。Cas9 核糖核蛋白可直接进行基因组编辑,但在递送方面仍存在挑战。对于黑色素瘤,透皮给药可将毒性降至最低,但面临皮肤屏障问题。我们设计了多功能脂质纳米颗粒(LNPs),用于靶向 Braf 基因递送 Cas9 RNP。通过金属微针预处理,我们的 LNPs 能有效编辑黑色素瘤细胞,减少 Braf 表达并抑制肿瘤生长。我们的研究证明了 LNPs 治疗黑色素瘤的潜力,并为癌症治疗中的高效体内 Cas9 RNP 递送系统铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


