Bone marrow transplantation increases sulfatase activity in somatic tissues in a multiple sulfatase deficiency mouse model
IF 5.4
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
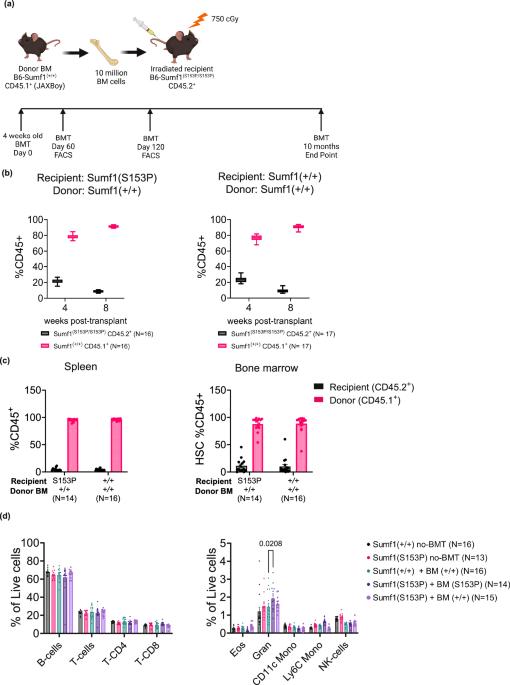
Multiple Sulfatase Deficiency (MSD) is an ultra-rare autosomal recessive disorder characterized by deficient enzymatic activity of all known sulfatases. MSD patients frequently carry two loss of function mutations in the SUMF1 gene, encoding a formylglycine-generating enzyme (FGE) that activates 17 different sulfatases. MSD patients show common features of other lysosomal diseases like mucopolysaccharidosis and metachromatic leukodystrophy, including neurologic impairments, developmental delay, and visceromegaly. There are currently no approved therapies for MSD patients. Hematopoietic stem cell transplant (HSCT) has been applied with success in the treatment of certain lysosomal diseases. In HSCT, donor-derived myeloid cells are a continuous source of active sulfatase enzymes that can be taken up by sulfatase-deficient host cells. Thus, HSCT could be a potential approach for the treatment of MSD. To test this hypothesis, we used a clinically relevant mouse model for MSD, B6-Sumf1(S153P/S153P) mice, engrafted with bone marrow cells, Sumf1+/+, from B6-PtprcK302E mice (CD45.1 immunoreactive). After 10 months post-transplant, flow cytometric analysis shows an average of 90% of circulating leukocytes of donor origin (Sumf1(+/+)). Enzymatic activity for ARSA, ARSB, and SGSH is significantly increased in spleen of B6-Sumf1(S153P/S153P) recipient mice. In non-lymphoid organs, only liver and heart show a significant correction of sulfatase activity and GAG accumulation. Frequency of inflammatory cells and lysosomal pathology is significantly reduced in liver and heart, while no significant improvement is detected in brain. Our results indicate that HSCT could be a suitable approach to treat MSD-pathology affecting peripheral organs, however that benefit to CNS pathology might be limited. Multiple Sulfatase Deficiency (MSD) is a rare genetic disorder caused by loss-of-function variations in the SUMF1 gene. This deficiency results in the accumulation of toxic compounds, leading to developmental delays and neurological impairments. In a bone marrow transplant (BMT), donor cells are infused into the patient and secrete active proteins that can help remove those toxic compounds. We carried out BMT in a mouse model for MSD and saw beneficial effects on peripheral organs, such as the liver and heart, but less change in neurological symptoms. Our results will be useful for the design of potential cell therapy approaches that could be used clinically to treat MSD. Presa et al. use a mouse model of multiple sulfatase deficiency (MSD) to assess efficacy of bone marrow transplantation to increase sulfatase activity. Sulfatase activity is partially restored in some peripheral tissues such as spleen, liver and heart, but no significant benefit is observed in the brain.
骨髓移植可提高多重硫酸酯酶缺乏症小鼠模型体细胞组织中硫酸酯酶的活性。
背景介绍多重硫酸酯酶缺乏症(MSD)是一种超罕见的常染色体隐性遗传疾病,其特征是所有已知硫酸酯酶的酶活性均不足。MSD 患者的 SUMF1 基因经常携带两个功能缺失突变,该基因编码一种甲酰甘氨酸生成酶(FGE),可激活 17 种不同的硫酸酶。MSD患者表现出其他溶酶体疾病(如粘多糖病和变色性白质营养不良症)的共同特征,包括神经系统损伤、发育迟缓和粘液性肥大。目前还没有针对MSD患者的获批疗法。造血干细胞移植(HSCT)已成功用于治疗某些溶酶体疾病。在造血干细胞移植中,捐献者髓系细胞是活性硫酸酯酶的持续来源,可被硫酸酯酶缺乏的宿主细胞吸收。因此,造血干细胞移植可能是治疗MSD的一种潜在方法:为了验证这一假设,我们使用了与临床相关的 MSD 小鼠模型--B6-Sumf1(S153P/S153P)小鼠,与来自 B6-PtprcK302E 小鼠(CD45.1 免疫反应阳性)的 Sumf1+/+ 骨髓细胞进行移植:移植后 10 个月,流式细胞仪分析显示,平均 90% 的循环白细胞来自供体来源(Sumf1(+/+))。B6-Sumf1(S153P/S153P)受体小鼠脾脏中 ARSA、ARSB 和 SGSH 的酶活性显著增加。在非淋巴器官中,只有肝脏和心脏的硫酸酯酶活性和 GAG 积累有明显的纠正。肝脏和心脏中的炎症细胞和溶酶体病变的频率明显降低,而脑部则没有发现明显改善:我们的研究结果表明,造血干细胞移植是治疗影响外周器官的MSD病理的一种合适方法,但对中枢神经系统病理的益处可能有限。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


