Personalised Health Behaviour Support Programme in Adults With Post-COVID Syndrome: A Randomised, Controlled Pilot Feasibility Trial
Abstract
Background
We investigated whether a novel 8-week personalised health behaviour support programme, focusing on the stability of symptoms and strategies to improve activities of daily living, was feasible and acceptable in adults with post-COVID syndrome.
Methods
In this randomised, controlled, pilot feasibility trial, 32 adults with post-COVID syndrome (continued symptoms for ≥ 12 weeks) were randomised 1:1 to receive personalised health behaviour support (self-reported physical activity and symptom diaries, plus seven one-to-one remotely delivered personalised self-management support sessions), once weekly for 8-weeks, or usual care (referral to online ‘your COVID-19 recovery’ programme). The primary outcome was the feasibility of recruiting and randomising adults with post-COVID syndrome. The secondary outcomes were to assess the acceptability and safety of the intervention and various outcome measures.
Results
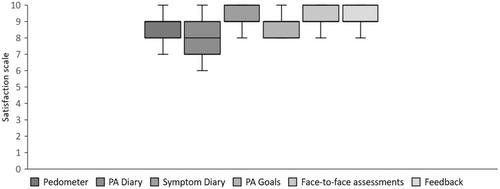
Of the 48 adults who expressed interest in the study, 32 (67%) were eligible and completed the baseline assessment. All 32 adults were willing to be randomised to either the personalised health behaviour support programme (n = 17) or usual care (n = 15) and 27 (age: 45 ± 12 years) adults completed follow-up at 9 weeks. The intervention was deemed feasible, with high adherence (92% and 94% completion rates for the physical activity and symptom diaries, respectively) and excellent acceptability rates (94% ‘liked the intervention a lot’). The intervention was deemed safe, with no symptom exacerbations reported.
Conclusion
An 8-week personalised health behaviour support programme was feasible for adults with post-COVID syndrome, with good adherence and acceptability rates. Early pilot data from this small sample also suggests meaningful improvements in physical activity, fatigue and respiratory symptoms.
Patient or Public Contribution
People living with post-COVID syndrome were involved from the outset with the study design, review of study documentation and interpretation of the data following completion. Furthermore, several participants have supported the local dissemination of findings following the completion of the study.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


