Single-cell transcriptomic analysis reveals dynamic activation of cellular signaling pathways regulating beige adipogenesis
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
PDGFRA+ cells have been identified as adipocyte stem cells (ASCs) that differentiate into beige adipocytes in white adipose tissue (WAT) following thermogenic stimuli. To elucidate the molecular heterogeneity of ASCs, we conducted single-cell transcriptomic profiling of PDGFRA+ cells isolated from the inguinal WAT (iWAT) of mice treated with the beta3 adrenergic receptor agonist CL316243. Single-cell RNA-seq revealed nine major clusters, which were categorized into four groups: resting, proliferating, differentiating, and adipogenic factor-expressing cells (AFECs). Trajectory analysis revealed sequential activation of molecular pathways, including the Hedgehog and Notch signaling pathways, during beige adipogenesis. AFECs expressed Dpp4 and did not differentiate into adipocytes in culture or after transplantation. Furthermore, genetic lineage tracing studies indicated that DPP4+ cells did not differentiate into adipocytes in iWAT during CL316243-induced beige adipogenesis. However, high-fat diet feeding led to the recruitment of adipocytes from DPP4+ cells in iWAT. Overall, this study improved our understanding of the dynamic molecular basis of beige adipogenesis and the potential contribution of DPP4+ adipocyte lineages to the pathological expansion of WAT during diet-induced obesity. This research examines beige adipogenesis, or the creation of ‘beige’ fat cells that burn energy and could help fight obesity. The scientists discovered a group of cells, identifed by specific markers PDGFRA and DPP4, which serve as a source for beige adipogenesis but don’t turn into beige fat cells themselves. They also found that these cells can change to become fat cells under certain situations, like a high-fat diet. The study also showed that the Hedgehog and Notch signaling pathways are vital in the transformation of PDGFRA+ cells into beige fat cells. These discoveries could be important for developing anti-obesity therapeutics. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
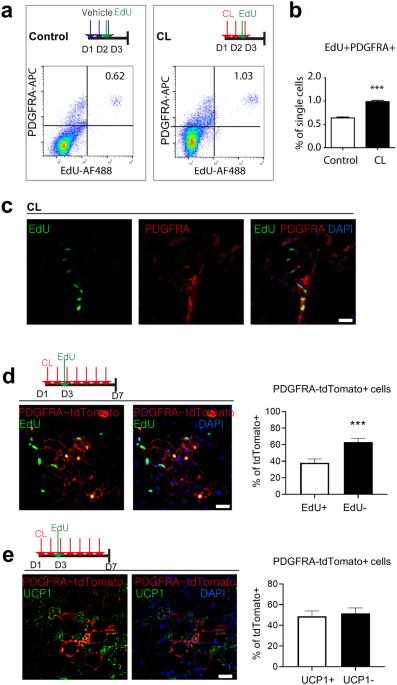
单细胞转录组分析揭示了调控米色脂肪生成的细胞信号通路的动态激活。
PDGFRA+细胞被鉴定为脂肪细胞干细胞(ASCs),在热刺激下可分化为白色脂肪组织(WAT)中的米色脂肪细胞。为了阐明ASCs的分子异质性,我们对从β3肾上腺素能受体激动剂CL316243处理的小鼠腹股沟WAT(iWAT)中分离出的PDGFRA+细胞进行了单细胞转录组分析。单细胞 RNA 序列分析发现了九个主要细胞群,并将其分为四组:静息细胞、增殖细胞、分化细胞和成脂因子表达细胞(AFECs)。轨迹分析显示,在米色脂肪生成过程中,包括刺猬和Notch信号通路在内的分子通路依次被激活。AFECs表达Dpp4,在培养或移植后不会分化成脂肪细胞。此外,遗传系谱追踪研究表明,在CL316243诱导的米色脂肪生成过程中,DPP4+细胞并没有分化成iWAT中的脂肪细胞。然而,高脂饮食会导致 iWAT 中的 DPP4+ 细胞募集成脂肪细胞。总之,这项研究提高了我们对米色脂肪生成的动态分子基础以及DPP4+脂肪细胞系对饮食诱导肥胖过程中WAT病理性扩张的潜在贡献的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


