Bee venom prompts the inhibition of gefitinib on proliferation, migration, and invasion of non-small cell lung cancer cells via EGFR-mediated autophagy
IF 2.6
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
It has been confirmed that bee venom (BV) can inhibit tumor metastasis of lung cancer cells induced by epidermal growth factor, suggesting the inhibitory role of BV on the regulation of epidermal growth factor receptor (EGFR), and may synergistically promote the anti-lung cancer effect of EGFR tyrosine kinase inhibitor gefitinib. This paper aims to ascertain the therapeutic potentials of BV combined with gefitinib against non-small cell lung cancer (NSCLC) in vitro. As results, the content of the main component melittin in air-dried BV was determined by HPLC. Subsequently, it was found that BV significantly inhibited the proliferation of NSCLC PC-9 and NCI-H1299 cells, but not generated apparent toxicity to human normal lung epithelial BEAS-2B cells. Meanwhile, the combination of BV and gefitinib also significantly inhibited the proliferation of these two cells, and suppressed the migration and invasion of PC-9 cells. By bioinformatics analysis and molecular docking, it was predicted that the main component melittin in BV could act on the cell membrane and transmembrane protein EGFR. Ultimately, Western blot assays showed BV alone or combined with gefitinib significantly decreased the protein expression of phosphorylated EGFR (p-EGFR) and the protein expression ratio of p-EGFR to EGFR, and increased the protein expression ratio of LC3-II to LC3-I in PC-9 cells or epidermal growth factor-activated PC-9 cells. The results demonstrated that BV could prompt the inhibition of gefitinib on proliferation, migration, and invasion of NSCLC cells via EGFR-mediated autophagy, showing the synergistic anti-NSCLC potential when combined with gefitinib.
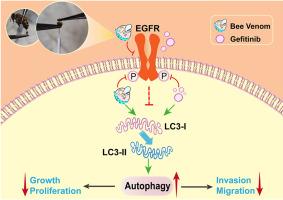
蜂毒通过表皮生长因子受体介导的自噬作用促使吉非替尼抑制非小细胞肺癌细胞的增殖、迁移和侵袭。
研究证实,蜂毒(BV)可抑制表皮生长因子诱导的肺癌细胞的肿瘤转移,提示BV对表皮生长因子受体(EGFR)具有抑制调控作用,并可协同促进EGFR酪氨酸激酶抑制剂吉非替尼的抗肺癌作用。本文旨在体外研究 BV 与吉非替尼联合治疗非小细胞肺癌(NSCLC)的潜力。结果,通过高效液相色谱法测定了风干 BV 中主要成分 Melittin 的含量。结果发现,BV能明显抑制NSCLC PC-9和NCI-H1299细胞的增殖,但对正常肺上皮细胞BEAS-2B没有明显毒性。同时,BV与吉非替尼联用也能明显抑制这两种细胞的增殖,并抑制PC-9细胞的迁移和侵袭。通过生物信息学分析和分子对接,预测BV中的主要成分melittin可作用于细胞膜和跨膜蛋白表皮生长因子受体。最终,Western 印迹检测显示,在 PC-9 细胞或表皮生长因子激活的 PC-9 细胞中,BV 单独或与吉非替尼联用可显著降低磷酸化表皮生长因子受体(p-EGFR)的蛋白表达量和 p-EGFR 与 EGFR 的蛋白表达比,并提高 LC3-II 与 LC3-I 的蛋白表达比。结果表明,BV能通过表皮生长因子受体介导的自噬作用促使吉非替尼对NSCLC细胞的增殖、迁移和侵袭产生抑制作用,与吉非替尼联合使用能显示出协同抗NSCLC的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
文献相关原料
公司名称
产品信息
上海源叶
GEF
上海源叶
MEL
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


