Single-cell chemoproteomics identifies metastatic activity signatures in breast cancer
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Protein activity state, rather than protein or mRNA abundance, is a biologically regulated and relevant input to many processes in signaling, differentiation, development, and diseases such as cancer. While there are numerous methods to detect and quantify mRNA and protein abundance in biological samples, there are no general approaches to detect and quantify endogenous protein activity with single-cell resolution. Here, we report the development of a chemoproteomic platform, single-cell activity-dependent proximity ligation, which uses automated, microfluidics-based single-cell capture and nanoliter volume manipulations to convert the interactions of family-wide chemical activity probes with native protein targets into multiplexed, amplifiable oligonucleotide barcodes. We demonstrate accurate, reproducible, and multiplexed quantitation of a six-enzyme (Ag-6) panel with known ties to cancer cell aggressiveness directly in single cells. We further identified increased Ag-6 enzyme activity across breast cancer cell lines of increasing metastatic potential, as well as in primary patient-derived tumor cells and organoids from patients with breast cancer.
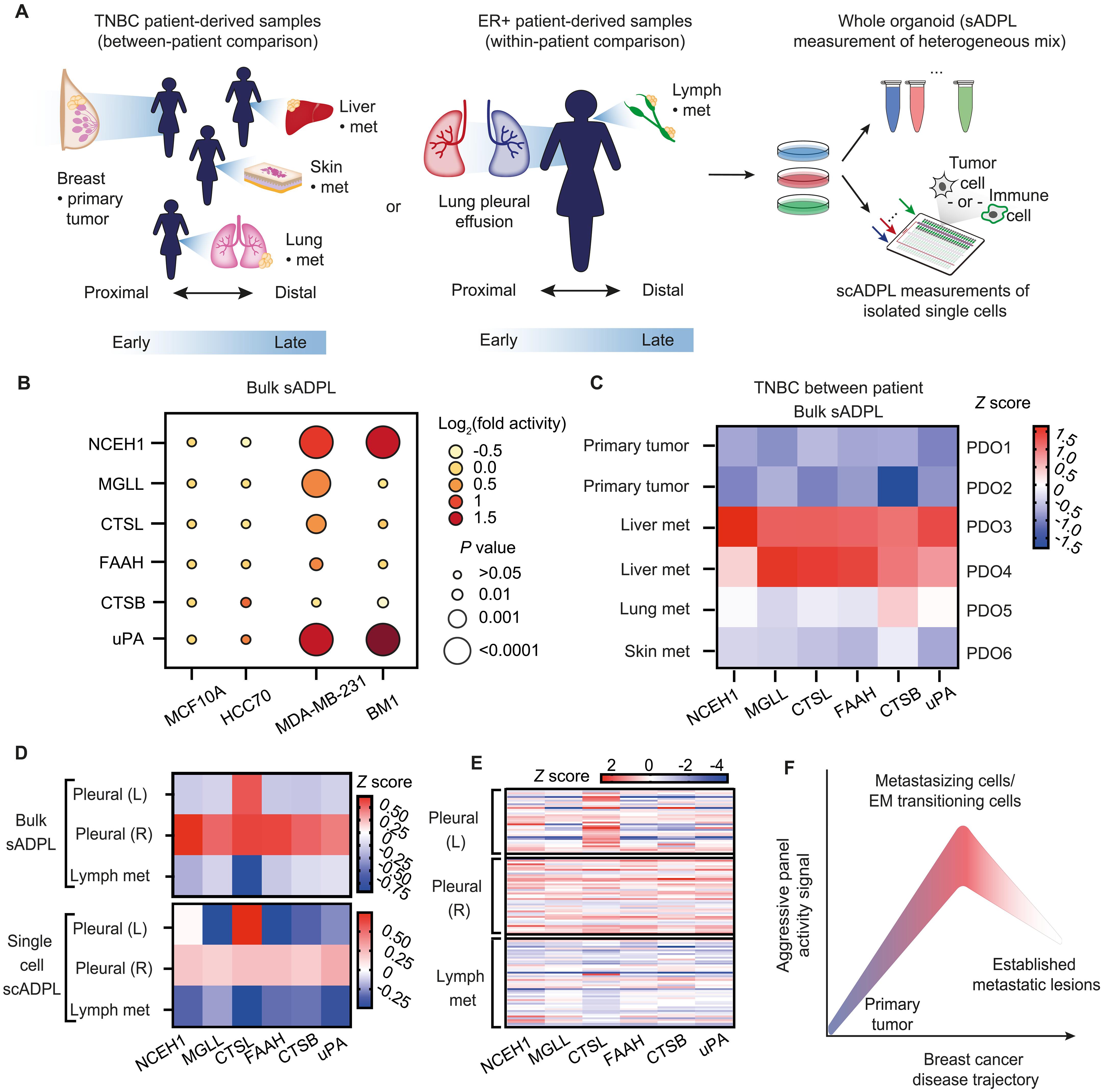
单细胞化学蛋白质组学识别乳腺癌的转移活动特征。
蛋白质活性状态,而不是蛋白质或 mRNA 丰度,是一种生物调控,与信号传导、分化、发育和癌症等疾病的许多过程相关。虽然有许多方法可以检测和量化生物样本中的 mRNA 和蛋白质丰度,但还没有一种通用方法可以检测和量化单细胞分辨率的内源性蛋白质活性。在这里,我们报告了化学蛋白质组学平台--单细胞活性依赖性邻近连接--的开发情况,该平台利用基于微流体技术的自动化单细胞捕获和纳升体积操作,将全家族化学活性探针与原生蛋白质靶标的相互作用转化为多路复用、可扩增的寡核苷酸条形码。我们展示了在单细胞中直接对已知与癌细胞侵袭性有关的六种酶(Ag-6)进行精确、可重复和多重定量的方法。我们进一步发现,在转移潜力不断增加的乳腺癌细胞系中,以及在乳腺癌患者的原发肿瘤细胞和器官组织中,Ag-6 酶的活性都在增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


