Fangchinoline suppresses hepatocellular carcinoma by regulating ROS accumulation via the TRIM7/Nrf2 signaling pathway
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Background
Dysregulation of redox homeostasis is associated with developing hepatocellular carcinoma (HCC). Oxidative stress (OS) is distinguished by the accumulation of ROS, which plays a variety of roles in cancer pathology. Fangchinoline (FAN), a bis-benzylisoquinoline alkaloid, has anti-cancer pharmacological activity. However, the regulatory mechanism of FAN on OS and whether it can inhibit HCC by mediating OS are still unclear.
Hypothesis/Purpose
This paper aims to explore the effectiveness of FAN in preventing HCC via regulating OS and identify the underlying molecular mechanisms.
Methods
We used the primary HCC mouse model and hepatoma cell line to explore the suppressive effect of FAN on hepatocarcinogenesis. To study the role of ROS in the anti-hepatocarcinoma effect of FAN in cell model and mouse model. The mechanism of FAN-induced nuclear factor erythroid 2-related factor 2 (Nrf2) pathway activation was studied through various techniques, including generation of Nrf2 and tripartite motif containing 7 (TRIM7) gene overexpressing or knockdown cell model, co-immunoprecipitation, immunohistochemistry and subcutaneous tumor xenograft models constructed by the stable TRIM7-overexpression HLE cells, etc.
Results
We showed that FAN significantly inhibited cell proliferation and hepatocarcinogenesis in HCC cells and primary HCC mouse model. The FAN-induced mitochondrial dysfunction promoted ROS accumulation, and using N-Acetylcysteine to clear ROS reversed the anti-HCC effects of FAN. We observed that FAN is capable of activating the Nrf2 pathway. This effect was thought to be due to the fact that, in response to the FAN-induced OS, the cancer cells created a feedback loop to stable Nrf2 via depressing the K48-linkage ubiquitination of it, which was caused by reduced binding of kelch-like ECH-associated protein 1 (Keap1) and Nrf2 and elevated TRIM7 expression. Indeed, overexpression of TRIM7 suppressed the anti-hepatocarcinoma effect of FAN.
Conclusion
The study determines the anti-liver cancer effect of FAN and first describes the positive regulatory effect of TRIM7 on Nrf2 signaling. We reveal that TRIM7/Nrf2 signaling served as a target of FAN-induced ROS accumulation in HCC, which helps to clarify the mechanism of action of FAN against HCC and provides a theoretical basis for FAN as an anti-cancer drug.
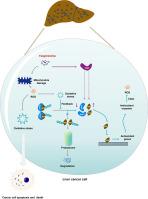
方棘霉素通过 TRIM7/Nrf2 信号通路调节 ROS 积累,从而抑制肝细胞癌。
背景:氧化还原平衡失调与肝细胞癌(HCC)的发生有关。氧化应激(OS)以 ROS 的积累为特征,在癌症病理学中发挥着多种作用。芳喹啉(FAN)是一种双苄基异喹啉生物碱,具有抗癌药理活性。假设/目的:本文旨在探讨方胆喹啉(FAN)通过调节 OS 预防 HCC 的有效性,并确定其潜在的分子机制:方法:我们利用原发性 HCC 小鼠模型和肝癌细胞系来探讨 FAN 对肝癌发生的抑制作用。在细胞模型和小鼠模型中研究 ROS 在 FAN 抗肝癌作用中的作用。通过多种技术,包括生成Nrf2和含三方基序7(TRIM7)基因过表达或敲除的细胞模型、共免疫沉淀、免疫组化和由稳定的TRIM7过表达HLE细胞构建的皮下肿瘤异种移植模型等,研究FAN诱导核因子红细胞2相关因子2(Nrf2)通路激活的机制。结果:我们发现 FAN 能显著抑制 HCC 细胞和原代 HCC 小鼠模型的细胞增殖和肝癌发生。FAN 诱导的线粒体功能障碍促进了 ROS 的积累,而使用 N-乙酰半胱氨酸清除 ROS 可逆转 FAN 的抗 HCC 作用。我们观察到 FAN 能够激活 Nrf2 通路。这种效应被认为是由于在FAN诱导的OS作用下,癌细胞通过抑制Nrf2的K48-连接泛素化,形成了一个稳定Nrf2的反馈回路,这是由kelch-like ECH-associated protein 1(Keap1)和Nrf2结合减少以及TRIM7表达升高引起的。事实上,TRIM7的过表达抑制了FAN的抗肝癌作用:该研究确定了 FAN 的抗肝癌作用,并首次描述了 TRIM7 对 Nrf2 信号转导的正向调节作用。我们发现 TRIM7/Nrf2 信号转导是 FAN 诱导 HCC 中 ROS 积累的靶点,这有助于阐明 FAN 对 HCC 的作用机制,并为 FAN 作为抗癌药物提供理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


