Arbuscular mycorrhizal symbiosis enhances the accumulation of plant-derived carbon in soil organic carbon by regulating the biosynthesis of plant biopolymers and soil metabolism
IF 6.1
2区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
Plant-derived carbon (C) is a critical constituent of particulate organic carbon (POC) and plays an essential role in soil organic carbon (SOC) sequestration. Yet, how arbuscular mycorrhizal fungi (AMF) control the contribution of plant-derived C to SOC storage through two processes (biosynthesis of plant biopolymers and soil metabolism) remains poorly understood. Here, we utilized transcriptome analysis to examine the effects of AMF on P. communis roots. Under the AM symbiosis, root morphological growth and tolerance to stress were strengthened, and the biosynthetic pathways of key plant biopolymers (long-chain fatty acids, cutin, suberin, and lignin) contributing to the plant-derived C were enhanced. In the subsequent metabolic processes, AMF increased soil metabolites contributing to plant-derived C (such as syringic acid) and altered soil metabolic pathways, including carbohydrate metabolism. Additionally, C-acquiring soil extracellular enzyme activities were enhanced by AMF, which could affect the stabilization of plant-derived C in soil. The contents of POC (21.71 g kg−1 soil), MAOC (10.75 g kg−1 soil), and TOC (32.47 g kg−1 soil) in soil were significantly increased by AMF. The concentrations of plant-derived C and microbial-derived C were quantified based on biomarker analysis. AMF enhanced the content of plant-derived C in both POC and MAOC fractions. What's more, plant-derived C presented the highest level in the POC fraction under the AMF treatment. This research broadens our understanding of the mechanism through which plant-derived C contributes to the accumulation of POC and SOC induced by AM symbiosis, and evidences the benefits of AMF application in SOC sequestration.
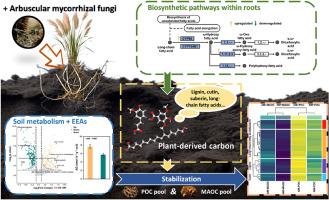
丛枝菌根共生通过调节植物生物聚合物的生物合成和土壤新陈代谢,提高了土壤有机碳中植物衍生碳的积累。
植物源碳(C)是颗粒有机碳(POC)的重要组成部分,在土壤有机碳(SOC)螯合中发挥着重要作用。然而,人们对丛枝菌根真菌(AMF)如何通过两个过程(植物生物聚合物的生物合成和土壤新陈代谢)控制植物源碳对土壤有机碳储存的贡献仍然知之甚少。在这里,我们利用转录组分析来研究 AMF 对 P. communis 根系的影响。在AM共生条件下,根系的形态生长和对胁迫的耐受性得到了增强,植物生物多聚物(长链脂肪酸、角质素、单宁和木质素)的生物合成途径也得到了加强,从而提高了植物源C。在随后的代谢过程中,AMF 增加了有助于植物源 C 的土壤代谢物(如丁香酸),并改变了土壤代谢途径,包括碳水化合物代谢。此外,AMF 还增强了土壤胞外酶获取 C 的活性,这可能会影响土壤中植物源 C 的稳定。土壤中 POC(21.71 g kg-1 土壤)、MAOC(10.75 g kg-1 土壤)和 TOC(32.47 g kg-1 土壤)的含量在 AMF 的作用下显著增加。根据生物标记分析,对植物源 C 和微生物源 C 的浓度进行了量化。AMF 提高了 POC 和 MAOC 部分中植物源 C 的含量。此外,在 AMF 处理下,POC 部分的植物源 C 含量最高。这项研究拓宽了我们对植物源C促进AM共生诱导的POC和SOC积累的机制的认识,并证明了应用AMF在SOC固碳中的益处。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.10
自引率
3.10%
发文量
410
审稿时长
33 days
期刊介绍:
Plant Physiology and Biochemistry publishes original theoretical, experimental and technical contributions in the various fields of plant physiology (biochemistry, physiology, structure, genetics, plant-microbe interactions, etc.) at diverse levels of integration (molecular, subcellular, cellular, organ, whole plant, environmental). Opinions expressed in the journal are the sole responsibility of the authors and publication does not imply the editors'' agreement.
Manuscripts describing molecular-genetic and/or gene expression data that are not integrated with biochemical analysis and/or actual measurements of plant physiological processes are not suitable for PPB. Also "Omics" studies (transcriptomics, proteomics, metabolomics, etc.) reporting descriptive analysis without an element of functional validation assays, will not be considered. Similarly, applied agronomic or phytochemical studies that generate no new, fundamental insights in plant physiological and/or biochemical processes are not suitable for publication in PPB.
Plant Physiology and Biochemistry publishes several types of articles: Reviews, Papers and Short Papers. Articles for Reviews are either invited by the editor or proposed by the authors for the editor''s prior agreement. Reviews should not exceed 40 typewritten pages and Short Papers no more than approximately 8 typewritten pages. The fundamental character of Plant Physiology and Biochemistry remains that of a journal for original results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


