Effect of conformational landscape on the polymorphism and monomorphism of tizanidine cocrystallization outcomes
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Molecular conformational diversity plays a crucial role in both polymorphic nucleation and cocrystal formation during the cocrystallization process. However, the relationship between molecular conformation and cocrystallization polymorphism is not well-explored. Herein, the impact of molecular conformational landscapes on cocrystallization outcomes was investigated using tizanidine (TZND) as model compound. Four coformers, namely maleic acid (MA), salicylic acid (SA), p-hydroxybenzoic acid (pHBA), and heptanedioic acid (HDA), were employed and five salt forms were developed for the first time. Compared with TZND, all five salts showed significantly improved water solubility and dissolution rate. The cocrystallization behavior of TZND varied with each coformer: MA exhibited solvent-dependent polymorphism, while SA, pHBA, and HDA showed solvent-independent monomorphism. Crystal structure and conformational analyses revealed the conformational variation of TZND across different cocrystallization outcomes. Molecular dynamics simulations and quantum chemical calculations demonstrated that the interplay between solvent effects and coformer interactions determines the dominant conformations of TZND. The cocrystallization nucleation process was also examined, and the molecular mechanism that explains both polymorphism and monomorphism in the cocrystallization of TZND was proposed.
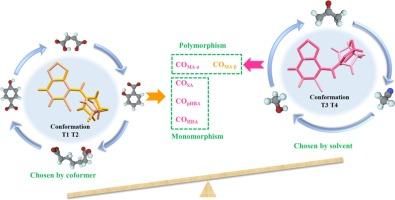
构象格局对替扎尼定共晶结果的多态性和单态性的影响。
分子构象多样性在共晶体形成过程中的多态成核和共晶体形成中起着至关重要的作用。然而,分子构象与结晶多态性之间的关系还没有得到很好的探讨。本文以替扎尼定(TZND)为模型化合物,研究了分子构象景观对结晶结果的影响。研究采用了马来酸(MA)、水杨酸(SA)、对羟基苯甲酸(pHBA)和庚二酸(HDA)四种共聚物,并首次开发了五种盐型。与 TZND 相比,这五种盐的水溶性和溶解速率都有明显提高。TZND 的共晶行为随每种共聚物的不同而变化:MA 表现出溶剂依赖性多态性,而 SA、pHBA 和 HDA 则表现出溶剂非依赖性单态性。晶体结构和构象分析揭示了 TZND 在不同共结晶结果下的构象变化。分子动力学模拟和量子化学计算表明,溶剂效应和共形作用之间的相互作用决定了 TZND 的主要构象。此外,还研究了共晶成核过程,并提出了解释 TZND 共晶过程中多态性和单态性的分子机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


