Study on the crystallinity of PEG on the crystalline size of flavonoids in a crystalline dispersion system
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-21
DOI:10.1016/j.ejpb.2024.114536
引用次数: 0
Abstract
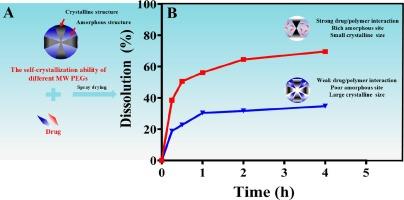
Poor water solubility and low bioavailability of flavonoids present significant barriers to their development and application. To address these challenges, this study explores the use of crystalline solid dispersions (CSDs) to reduce drug crystalline size and enhance in vivo bioavailability. The CSDs were prepared using a spray-drying technique with chrysin (CHY) and quercetin (QUR) as model drugs and various molecular weights of polyethylene glycol (PEG) as carriers. The authors systematically investigated the factors influencing the interaction between flavonoids and PEG in CSDs. These factors included the relationships between intermolecular interactions and PEG molecular weight, crystallinity, microstructures such as crystalline domain size and crystal morphology of the flavonoids and PEG in CSDs, crystalline size of the drug in CSDs, and in vitro dissolution rate and in vivo pharmacokinetics. Our results indicated that the interaction between flavonoids and PEG in CSDs was influenced more by PEG crystallinity than by its molecular weight. Lower crystallinity of PEG, achieved through recrystallization, led to stronger intermolecular interactions with the drugs. Specifically, PEG8000 exhibited the lowest crystallinity, indicating a higher content of PEG in the amorphous state, which interacted more effectively with the amorphous drug in CSDs. This interaction significantly inhibited drug crystallization growth, resulting in a marked decrease in drug crystalline domain size and crystalline size. Consequently, PEG8000 was identified as the optimal carrier for preparing CSDs, achieving the best cumulative dissolution percentage. The QUR/PEG8000-CSD formulation increased the cumulative dissolution percentage and oral bioavailability of QUR by 18.76 and 20.66 times, respectively, compared to QUR alone. This study demonstrates that PEG crystallinity, following recrystallization, directly affects its intermolecular interactions with the drug, thereby impacting drug crystalline size and dissolution rate.
研究 PEG 的结晶度对结晶分散体系中黄酮类化合物结晶尺寸的影响。
黄酮类化合物的水溶性差、生物利用率低,是其开发和应用的重大障碍。为了应对这些挑战,本研究探讨了使用结晶固体分散体(CSDs)来减小药物结晶尺寸并提高体内生物利用度的方法。研究人员采用喷雾干燥技术,以菊黄素(CHY)和槲皮素(QUR)为模型药物,以不同分子量的聚乙二醇(PEG)为载体制备了 CSDs。作者系统地研究了影响 CSD 中黄酮类化合物与 PEG 之间相互作用的因素。这些因素包括分子间相互作用与 PEG 分子量之间的关系、结晶度、微结构(如 CSD 中黄酮类化合物和 PEG 的结晶畴尺寸和晶体形态)、CSD 中药物的结晶尺寸、体外溶出率和体内药代动力学。我们的研究结果表明,黄酮类化合物和 PEG 在 CSDs 中的相互作用受 PEG 结晶度的影响比受其分子量的影响更大。通过重结晶获得的 PEG 结晶度越低,与药物的分子间相互作用就越强。具体来说,PEG8000 的结晶度最低,表明无定形状态下的 PEG 含量较高,能更有效地与 CSD 中的无定形药物相互作用。这种相互作用极大地抑制了药物结晶的生长,导致药物结晶畴尺寸和结晶尺寸明显减小。因此,PEG8000 被认为是制备 CSD 的最佳载体,其累积溶出率最高。与单独使用 QUR 相比,QUR/PEG8000-CSD 制剂的累积溶出率和口服生物利用度分别提高了 18.76 倍和 20.66 倍。这项研究表明,PEG 重结晶后的结晶度会直接影响其与药物的分子间相互作用,从而影响药物的结晶大小和溶出率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


