Evaluating surfactant effectiveness in preventing antibody adsorption directly on medical surfaces using a novel device
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-20
DOI:10.1016/j.ejpb.2024.114539
引用次数: 0
Abstract
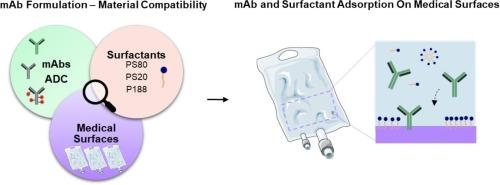
Biopharmaceuticals, specifically antibody-based therapeutics, have revolutionized disease treatment. Throughout their lifecycle, these therapeutic proteins are exposed to several stress conditions, for example at interfaces, posing a risk to the drug product stability, safety and quality. Therapeutic protein adsorption at interfaces may lead to loss of active product and protein aggregation, with potential immunogenicity risks. Non-ionic surfactants are commonly added in formulations to mitigate protein-surface interactions. However, their effectiveness varies with the monoclonal antibody (mAb), and model surface material. Extrapolating findings from model surfaces to real medical surfaces is challenging due to diverse properties.
This study pioneers the evaluation of surfactant effectiveness in preventing mAb adsorption directly on medical surfaces at the medical bag/formulation interface, utilizing the ELIBAG device. The adsorption of different protein modalities, mAbs and antibody-drug conjugate (ADC), using three surfactants (PS80, PS20, and P188), was examined across various medical surfaces, IV bags and manufacturing bags, and model surfaces. Our findings reveal that surfactants prevent mAb adsorption depending on the mAb modality, surfactant type and concentration, and surface material. This research underscores the importance of considering real medical surfaces in direct contact with formulations, offering insights for enhancing drug product development and ensuring material-protein compatibility in real world use.
使用新型装置评估表面活性剂在防止抗体直接吸附在医疗表面上的有效性。
生物制药,特别是以抗体为基础的疗法,为疾病治疗带来了革命性的变化。在整个生命周期中,这些治疗蛋白质会暴露在多种应力条件下,例如在界面上,从而对药物产品的稳定性、安全性和质量构成风险。治疗蛋白质在界面上的吸附可能会导致活性产品流失和蛋白质聚集,并带来潜在的免疫原性风险。配方中通常会添加非离子表面活性剂,以减轻蛋白质与表面的相互作用。然而,非离子表面活性剂的效果因单克隆抗体(mAb)和模型表面材料而异。由于模型表面的特性各不相同,因此将模型表面的研究结果推广到真实的医疗表面具有挑战性。本研究利用 ELIBAG 设备,率先评估了表面活性剂在医疗袋/制剂界面上直接防止 mAb 吸附的有效性。研究人员使用三种表面活性剂(PS80、PS20 和 P188)在不同的医疗表面、静脉注射袋和制造袋以及模型表面对不同蛋白质模式(mAb 和抗体药物结合物 (ADC))的吸附情况进行了检测。我们的研究结果表明,表面活性剂能阻止 mAb 吸附取决于 mAb 模式、表面活性剂类型和浓度以及表面材料。这项研究强调了考虑与制剂直接接触的真实医疗表面的重要性,为加强药物产品开发和确保材料-蛋白质在实际使用中的兼容性提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


