Towards the understanding of the IVPT results variability—Development, verification and validation of the PBPK model of caffeine in vitro human skin permeation
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
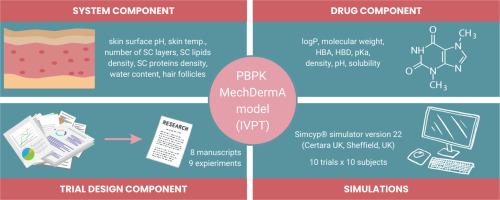
In the context of evaluating the safety and efficacy of dermal products, pharmacokinetic (PK) studies face considerable challenges, particularly concerning topically applied formulations. This underscores the necessity for alternative methods, such as in vitro permeation tests (IVPT) and physiologically based pharmacokinetic (PBPK) modelling, to better understand the dermal pharmacokinetics of a product. The purpose of this study was to modify, verify, and validate the PBPK model of caffeine permeation through human skin previously developed by Patel et al. (2022), and compare simulation results with experimental data from IVPT studies. Moreover, the study aimed to analyse the IVPT data variability and explore the potential of using the PBPK model to understand the influence of biological and drug-related factors on the IVPT results. In total, eight manuscripts describing nine experiments were included. The overall shapes of the permeation curves were considered acceptable based on visual checks for all analysed experiments. Five out of nine experiments met the predefined standard 2-fold difference criterion for comparison of the cumulative amount of caffeine in the receptor solution.. Our investigation highlights challenges in validating PBPK models for IVPT experiments, as the quality and consistency of experimental results pose significant hurdles. Despite access to data on caffeine permeation in scientific literature, reliable model validation is currently infeasible. Inter-laboratory variation suggests that alternative validation methods may be needed. Further studies should focus on issues with other compounds, especially lipophilic ones.
了解 IVPT 结果的变异性--咖啡因体外人体皮肤渗透 PBPK 模型的开发、验证和确认。
在评估皮肤产品的安全性和有效性方面,药代动力学(PK)研究面临着相当大的挑战,特别是在局部应用制剂方面。这凸显了采用体外渗透试验(IVPT)和生理药代动力学(PBPK)建模等替代方法来更好地了解产品的皮肤药代动力学的必要性。本研究的目的是修改、验证和确认 Patel 等人(2022 年)之前开发的咖啡因通过人体皮肤渗透的 PBPK 模型,并将模拟结果与 IVPT 研究的实验数据进行比较。此外,该研究还旨在分析 IVPT 数据的变异性,并探索使用 PBPK 模型了解生物和药物相关因素对 IVPT 结果影响的潜力。本研究共收录了 8 篇手稿,描述了 9 项实验。根据对所有分析实验的目测检查,认为渗透曲线的整体形状是可以接受的。九项实验中有五项达到了预定的标准 2 倍差异标准,以比较受体溶液中咖啡因的累积量。我们的调查凸显了为 IVPT 实验验证 PBPK 模型所面临的挑战,因为实验结果的质量和一致性构成了重大障碍。尽管可以从科学文献中获得咖啡因渗透的数据,但可靠的模型验证目前还不可行。实验室之间的差异表明,可能需要采用其他验证方法。进一步的研究应关注其他化合物,尤其是亲脂性化合物的问题。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


