Copper-coordinated nanomedicine for the concurrent treatment of lung cancer through the induction of cuproptosis and apoptosis
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The resistance of tumor cells to apoptosis often leads to chemoresistance and treatment failure in clinic. In this study, we have developed a Cu2+-coordinated lignosulfonate (CLS) /doxorubicin (DOX) biological complex (referred to as LCD) with the aim of overcoming cellular resistance to apoptosis for combined lung cancer therapy. The copper complexes modified by CLS exhibit significant water solubility and excellent in vivo biocompatibility. The proportion of copper in the composite is simultaneously increased. Due to the coordination and π-π stacking effects, the self-assembled LCD exhibits nanometer-scale particle size, a narrow and homogeneous grain distribution, as well as excellent dispersion stability. Furthermore, LCD has the potential to disassemble in the presence of high levels of glutathione (GSH) and low pH, leading to effective drug release. Cu2+-mediated cuproptosis can lead to the down-regulation of FDX1 and DLAT protein expression by reducing mitochondrial membrane potential, resulting in non-apoptotic programmed cell death (PCD) regardless of cellular resistance to apoptosis. Moreover, the released DOX not only exhibits a preference for localizing in the cell nucleus to induce apoptosis for combined chemotherapy, but also generates a substantial amount of H2O2. This H2O2 further produces ROS to induce apoptosis through Fenton reaction with Cu2+. LCD demonstrates significant superiority over monotherapy in inhibiting tumor growth while minimizing systemic toxicity through the combined action of cuproptosis and apoptosis. This study may provide a potential avenue for the advancement of self-delivery nanomedicine to overcome resistance to apoptosis in tumor therapy.
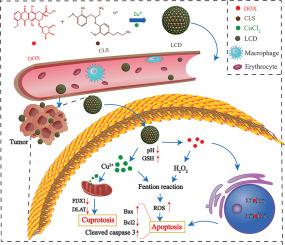
通过诱导杯突酶和细胞凋亡同时治疗肺癌的铜配位纳米药物。
肿瘤细胞对凋亡的抵抗往往导致化疗抗药性和临床治疗失败。在这项研究中,我们开发了一种 Cu2+ 配位木质素磺酸盐(CLS)/多柔比星(DOX)生物复合物(简称 LCD),旨在克服细胞对凋亡的耐药性,用于肺癌的联合治疗。经 CLS 修饰的铜复合物具有显著的水溶性和良好的体内生物相容性。铜在复合材料中的比例也同时增加。由于配位和π-π堆叠效应,自组装液晶显示出纳米级的粒度、窄而均匀的晶粒分布以及优异的分散稳定性。此外,LCD 还具有在高浓度谷胱甘肽(GSH)和低 pH 值条件下分解的潜力,从而实现有效的药物释放。Cu2+ 介导的杯突症可通过降低线粒体膜电位导致 FDX1 和 DLAT 蛋白表达下调,从而导致非凋亡性程序性细胞死亡(PCD),而不考虑细胞对凋亡的抵抗力。此外,释放出的 DOX 不仅偏好定位于细胞核内诱导细胞凋亡以进行联合化疗,还会产生大量的 H2O2。H2O2 与 Cu2+ 发生 Fenton 反应,进一步产生 ROS,诱导细胞凋亡。通过杯突酶和细胞凋亡的联合作用,LCD 在抑制肿瘤生长的同时最大限度地降低了全身毒性,在抑制肿瘤生长方面明显优于单一疗法。这项研究可能会为自传递纳米药物的发展提供一个潜在的途径,以克服肿瘤治疗中的凋亡耐药性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


