Neuroprotective Role of AQP4 Knockdown in Astrocytes After Oxygen–Glucose Deprivation
Abstract
Background
Aquaporin-4 (AQP4), predominantly expressed in astrocytes, has been implicated in the development of brain edema following ischemic events. However, its role in post-stroke neuroinflammation is not fully understood.
Methods
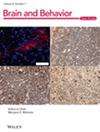
Using a middle cerebral artery occlusion (MCAO) mouse model, we assessed AQP4's role in post-stroke inflammation. Brain tissue slices from male C57BL/6 mice were subjected to immunohistochemistry and western blot post-MCAO. Additionally, primary astrocytes were isolated for quantitative real-time PCR and immunofluorescence assays to evaluate the expression of inflammatory markers glial fibrillary acidic protein (GFAP) and AQP4. AQP4 modulation was achieved using viral knockdown and overexpression methods. Neuronal damage was assessed using flow cytometry and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) tests in co-culture studies.
Results
MCAO mice exhibited a significant upregulation in GFAP. This reactive astrogliosis corresponded with an elevation in inflammatory markers. AQP4 expression responded to this inflammatory trend, peaking at 6 h after OGD and returning to baseline levels at 24 and 48 h. Co-culture experiments revealed that AQP4(+) astrocytes exacerbated injury in OGD-treated neurons, as evidenced by increased TUNEL positivity and apoptotic events. Conversely, AQP4(−) astrocytes appeared to have a protective effect. Knockdown of AQP4 resulted in reduced post-OGD inflammatory response, whereas AQP4 overexpression intensified the injury to neurons post-OGD. In vivo experiments also confirmed that AQP4 inhibitor TGN-020 reduced and overexpression of AQP4 increased behavioral abnormalities and brain infarcts.
Conclusion
Our findings underscore AQP4's pivotal role in modulating post-stroke neuroinflammation. Targeting AQP4 may present a novel therapeutic avenue for mitigating ischemia-induced neuronal damage.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


