Development and validation of a new and rapid molecular diagnostic tool based on RT-LAMP for Hepatitis C virus detection at point-of-care
IF 4.2
3区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Purpose
Globally, it is estimated that 1.0 million individuals are newly infected by Hepatitis C virus (HCV) every year, and nearly 50 million people live with a chronic infection, according to World Health Organization. To overcome underdiagnosis of HCV infection among hard-to-reach populations, it is essential to develop new rapid and easy-to-use molecular diagnostic systems. In this work, we have developed a pangenotypic diagnostic tool based on Loop-Mediated Isothermal Amplification (LAMP), coupled to a direct sample lysis procedure for molecular detection of HCV at point-of-care (POC).
Methods
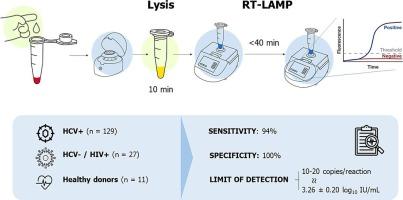
Procedure validation was performed using 129 different samples from HCV infected patients (116 serum samples, and 13 fresh blood samples), 27 individuals who tested negative for HCV but positive for HIV, and 11 healthy donors. Serum was collected, lysed for 10 min at room temperature, and assayed by RT-LAMP. To achieve this, a set of 9 LAMP-primers was used for the first time. Parallel RT-qPCR assays were conducted for HCV to both validate the procedure and quantify viral loads.
Results
HCV was detected by RT-LAMP in 109/116 HCV positive serum samples, and in 11/13 positive blood samples in less than 40 min. Compared to RT-qPCR results, our RT-LAMP procedure showed a sensitivity of 94 %, 100 % specificity, and a limit of detection of 3.26 log10 IU/mL (10–20 copies per reaction).
Conclusions
We have developed an accurate system, more affordable than the current available rapid tests for HCV. Since no prior RNA purification step from capillary blood is required, we strongly recommend our RT-LAMP system as a valuable and rapid tool for the molecular detection of HCV at POC.
开发并验证基于 RT-LAMP 的新型快速分子诊断工具,用于在护理点检测丙型肝炎病毒。
目的:据世界卫生组织估计,全球每年有 100 万人新感染丙型肝炎病毒(HCV),近 5000 万人患有慢性感染。为了解决丙型肝炎病毒感染在难以接触人群中诊断不足的问题,必须开发新的快速、易用的分子诊断系统。在这项工作中,我们开发了一种基于环路介导等温扩增(LAMP)的泛基因型诊断工具,并将其与直接样本裂解程序相结合,用于在护理点(POC)对 HCV 进行分子检测:方法:使用 129 份不同的样本(116 份血清样本和 13 份新鲜血液样本)进行了程序验证,这些样本分别来自 HCV 感染者、27 名 HCV 检测阴性但 HIV 检测阳性者和 11 名健康捐献者。采集血清后,在室温下裂解 10 分钟,然后用 RT-LAMP 进行检测。为此,首次使用了一套 9 个 LAMP-引物。同时还对 HCV 进行了 RT-qPCR 检测,以验证该程序并量化病毒载量:结果:在不到 40 分钟的时间内,RT-LAMP 法检测了 109/116 份 HCV 阳性血清样本和 11/13 份阳性血液样本中的 HCV。与 RT-qPCR 结果相比,我们的 RT-LAMP 程序的灵敏度为 94%,特异性为 100%,检测限为 3.26 log10 IU/mL(每次反应 10-20 个拷贝):结论:我们开发了一种准确的系统,比目前可用的 HCV 快速检测方法更经济实惠。由于无需事先从毛细管血液中纯化 RNA,我们强烈推荐我们的 RT-LAMP 系统,它是在 POC 上进行 HCV 分子检测的重要而快速的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Methods
生物-生化研究方法
CiteScore
9.80
自引率
2.10%
发文量
222
审稿时长
11.3 weeks
期刊介绍:
Methods focuses on rapidly developing techniques in the experimental biological and medical sciences.
Each topical issue, organized by a guest editor who is an expert in the area covered, consists solely of invited quality articles by specialist authors, many of them reviews. Issues are devoted to specific technical approaches with emphasis on clear detailed descriptions of protocols that allow them to be reproduced easily. The background information provided enables researchers to understand the principles underlying the methods; other helpful sections include comparisons of alternative methods giving the advantages and disadvantages of particular methods, guidance on avoiding potential pitfalls, and suggestions for troubleshooting.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


