Quantification of tricyclic glycopeptide in human plasma by UHPLC-MS3 coupled with counter-extraction follow by protein precipitation to enhance sensitivity
IF 2.8
3区 医学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
An ultra-high performance liquid chromatography tandem mass spectrometry cubed (UHPLC/MS3) assay coupled with protein precipitation and counter-extraction for detection of tricyclic glycopeptide vancomycin in human plasma was established and validated in this study. After protein precipitation and counter-extraction with dichloromethane, chromatographic separation of vancomycin and norvancomycin were performed on a reversed phase column (XBridge Peptide BEH C18 column, 2.1 × 100 mm I.D, 3.5 μm). The transition (parent ions → fragment ions → further fragment ions) at m/z 725.3 → 144.1 → 100.1 was used for quantification of vancomycin. The transition (parent ions → fragment ions) at m/z 718.3 → 144.2 was used for detection of norvancomycin. The linear range of the developed analytical method for quantification of vancomycin was 0.5–100 µg/mL (r = 0.9989). The range of intra- and inter-day precisions of the assay among low, medium and high concentrations is between 1.88 % and 6.33 %. The sensitivity of the analytical method was significantly improved by using MS3 technique as monitoring mode and counter-extraction with dichloromethane followed by protein precipitation as sample processing assay. The developed UHPLC/MS3 assay was successfully applied for clinical therapeutic drug monitoring (TDM) of vancomycin in 45 human plasma samples.
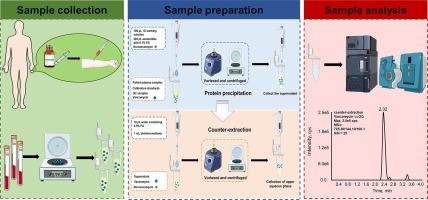
利用超高效液相色谱-质谱联用仪(UHPLC-MS3)和反萃取-蛋白质沉淀法提高灵敏度,对人血浆中的三环糖肽进行定量。
本研究建立并验证了一种超高效液相色谱-串联质谱立方(UHPLC/MS3)检测方法,该方法结合了蛋白沉淀和反萃取,用于检测人体血浆中的三环糖肽万古霉素。蛋白沉淀和二氯甲烷反萃取后,在反相色谱柱(XBridge Peptide BEH C18 column, 2.1 × 100 mm I.D, 3.5 μm)上分离万古霉素和诺万古霉素。用 m/z 725.3 → 144.1 → 100.1 的转变(母离子 → 片段离子 → 进一步的片段离子)对万古霉素进行定量。在 m/z 718.3 → 144.2 处的转换(母离子 → 片段离子)用于检测去万古霉素。所开发的万古霉素定量分析方法的线性范围为 0.5-100 µg/mL (r = 0.9989)。低、中、高浓度测定的日内和日间精确度范围为 1.88 % 至 6.33 %。采用 MS3 技术作为监测模式,并用二氯甲烷反萃取后沉淀蛋白质作为样品处理方法,大大提高了分析方法的灵敏度。所开发的超高效液相色谱/MS3测定法成功地应用于45份人体血浆样品中万古霉素的临床治疗药物监测(TDM)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chromatography B
医学-分析化学
CiteScore
5.60
自引率
3.30%
发文量
306
审稿时长
44 days
期刊介绍:
The Journal of Chromatography B publishes papers on developments in separation science relevant to biology and biomedical research including both fundamental advances and applications. Analytical techniques which may be considered include the various facets of chromatography, electrophoresis and related methods, affinity and immunoaffinity-based methodologies, hyphenated and other multi-dimensional techniques, and microanalytical approaches. The journal also considers articles reporting developments in sample preparation, detection techniques including mass spectrometry, and data handling and analysis.
Developments related to preparative separations for the isolation and purification of components of biological systems may be published, including chromatographic and electrophoretic methods, affinity separations, field flow fractionation and other preparative approaches.
Applications to the analysis of biological systems and samples will be considered when the analytical science contains a significant element of novelty, e.g. a new approach to the separation of a compound, novel combination of analytical techniques, or significantly improved analytical performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


