Neutrophil-derived IL-10 increases CVB3-induced acute pancreatitis pathology via suppressing CD8+T cell activation while increasing macrophage STAT3-IL-6 cascade
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Acute pancreatitis (AP) is a lethal inflammatory disease of the pancreas. Its pathogenesis remains obscure and specific treatments are lacking. An increase in Interleukin-10 (IL-10) in the early stage of AP patients is closely related to AP severity. In Coxsackievirus B3 (CVB3) induced murine AP model, we found early IL-10 increased viral replication and pancreatic inflammation, yet the cellular source of IL-10 and the immunomodulatory role of neutrophils during viral infection remains unknown. Here we show that CVB3 infection enhanced neutrophil infiltration and IL-10 expression in the pancreas at day3 post infection (p.i.). Neutrophils served as an important early source of pancreatic IL-10 at the initiation of infection. Day3 pancreas extracts (D3P) also induced bone-marrow derived neutrophils (BMneu) to secrete IL-10. Adoptive transfer of D3P-pretreated BMneu into IL-10 KO mice increased viral replication and pancreas histopathology, which effect was blunted by the absence of IL-10 in BMneu. Mechanically, IL-10+ neutrophil increased IL-10R1 expression on MΦs and activated STAT3-IL-6/IL-10 signaling cascade while decreased IL-12 and MHC II expression in MΦs, thus impairing IFN-γ+Granzyme B+CD8+T cell activation and viral clearance. Adoptive transferring infected mice with activated CD8+T cells 4 days p.i. attenuated viral load and AP pathology indicating an AP-protective effect. Our findings document a novel immunoregulatory function of neutrophils in acute CVB3 infection, in which neutrophil-derived IL-10 impairs anti-viral CD8+T activation, and amplifies intrapancreatic inflammation via activating MΦ STAT3-IL-6 signaling cascade. An IL-10-targeting option is suggested for the future treatment of viral AP.
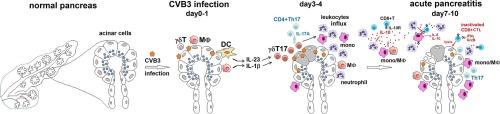
中性粒细胞衍生的IL-10可抑制CD8+T细胞活化,同时增加巨噬细胞STAT3-IL-6级联,从而增加CVB3诱导的急性胰腺炎病理变化。
急性胰腺炎(AP)是一种致命的胰腺炎症性疾病。其发病机制仍不明确,也缺乏特效治疗方法。急性胰腺炎患者早期白细胞介素-10(IL-10)的增加与急性胰腺炎的严重程度密切相关。在柯萨奇病毒 B3(CVB3)诱导的小鼠 AP 模型中,我们发现早期 IL-10 会增加病毒复制和胰腺炎症,但 IL-10 的细胞来源以及中性粒细胞在病毒感染过程中的免疫调节作用仍不清楚。在这里,我们发现CVB3感染后第3天(p.i.),中性粒细胞浸润和IL-10在胰腺中的表达增强。在感染开始时,中性粒细胞是胰腺IL-10的重要早期来源。第3天的胰腺提取物(D3P)还能诱导骨髓衍生的中性粒细胞(BMneu)分泌IL-10。将经D3P处理的骨髓中性粒细胞移植到IL-10 KO小鼠体内会增加病毒复制和胰腺组织病理学,而骨髓中性粒细胞中缺乏IL-10会减弱这种效应。从机制上讲,IL-10+中性粒细胞增加了MΦs上IL-10R1的表达,激活了STAT3-IL-6/IL-10信号级联,同时降低了MΦs上IL-12和MHC II的表达,从而损害了IFN-γ+粒酶B+CD8+T细胞的活化和病毒清除。用活化的CD8+T细胞于4天后收养转移感染小鼠,可减轻病毒载量和AP病理变化,这表明AP具有保护作用。我们的研究结果证明了中性粒细胞在CVB3急性感染中的新型免疫调节功能,其中中性粒细胞衍生的IL-10会损害CD8+T的抗病毒活化,并通过激活MΦ STAT3-IL-6信号级联放大胰腺内炎症。这为未来治疗病毒性 AP 提出了一种 IL-10 靶向方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


