Albumins constrainting the conformation of mitochondria-targeted photosensitizers for tumor-specific photodynamic therapy
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Tumor ablation Preclinical organelle-targeted phototherapies have effectively achieved tumor photoablation for regenerative biomedical applications in cancer therapies. However, engineering effective phototherapy drugs with precise tumor-localization targeting and organelle direction remains challenging. Herein, we report a albumins constrainting mitochondrial-targeted photosensitizer nanoparticles (PSs@BSAs) for tumor-specific photodynamic therapy. X-ray crystallography elucidates the two-stage assembly mechanism of PSs@BSAs. Femtosecond transient absorption spectroscopy and quantum mechanical calculations reveal the implications of conformational dynamics at the excited state. PSs@BSAs can efficiently disable mitochondrial activity, and further disrupt tumor angiogenesis based on the photodynamic effect. This triggers a metabolic and oxidative stress crisis to facilitate photoablation of solid tumor and antitumor metastasis. The study fully elucidates the interdisciplinary issues of chemistry, physics, and biological interfaces, thereby opening new horizons to inspire the engineering of organelle-targeted tumor-specific photosensitizers for biomedical applications.
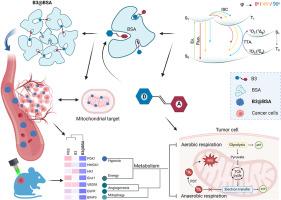
抑制线粒体靶向光敏剂构象的白蛋白,用于肿瘤特异性光动力疗法。
肿瘤消融 临床前细胞器靶向光疗法有效地实现了肿瘤光消融,可用于癌症治疗的再生生物医学应用。然而,设计具有精确的肿瘤定位靶向和细胞器定向的有效光疗药物仍然具有挑战性。在此,我们报告了一种用于肿瘤特异性光动力疗法的线粒体靶向光敏剂纳米颗粒(PSs@BSA)。X 射线晶体学阐明了 PSs@BSAs 的两阶段组装机制。飞秒瞬态吸收光谱和量子力学计算揭示了激发态构象动力学的影响。PSs@BSAs 能有效抑制线粒体活性,并在光动力效应的基础上进一步破坏肿瘤血管生成。这将引发新陈代谢和氧化应激危机,从而促进实体瘤的光消融和抗肿瘤转移。该研究充分阐明了化学、物理学和生物学界面的跨学科问题,从而为细胞器靶向肿瘤特异性光敏剂的生物医学应用工程开辟了新的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


