Potential mechanisms of metabolic reprogramming induced by ischemia–reperfusion injury in diabetic myocardium
Abstract
Objective
This study aimed to explore metabolic reprogramming in diabetic myocardium subjected to ischemia–reperfusion injury (I/RI) and potential mechanisms.
Background
Increased vulnerability after I/RI in diabetic myocardium is a major cause of the high prevalence of perioperative adverse cardiac events, and the specific alterations in energy metabolism after I/RI in diabetic myocardium and the impact on increased vulnerability are not fully understood.
Methods
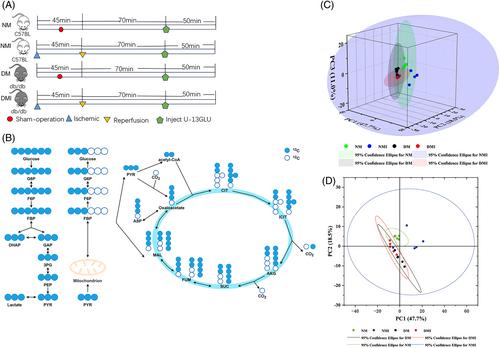
Metabolomic methods were used to explore the differences and characteristics of metabolites in the heart tissues of four groups, and then, single-cell RNA sequencing (ScRNA-seq) was used to explore the potential mechanism of metabolic reprogramming.
Results
It was found that the fatty acid metabolism of db/db mouse I/RI (DMI) showed a significant upward trend, especially the metabolites of ultra-long and medium-long-chain fatty acids; the metabolic flow analysis found that the U-13C glucose M + 6 was significantly higher in the C57BL mouse sham operation (NM) group than in the db/db mouse sham operation (DM) group, and in the C57BL mouse I/RI (NMI) than in the DMI group. Compared with the NMI group, the intermediate metabolites of glycolysis and tricarboxylic acid (TCA) cycle were significantly reduced in the DMI group; all comparisons were statistically significant (p < 0.05), indicating that the glucose uptake of diabetic myocardetis, the ability of glucose glycolysis after I/RI, and the contribution of glucose to TCA were significantly reduced. The results of ScRNA-seq revealed that the number of Cluster 0 myocardial isoforms was significantly increased in diabetic myocardium, and the differential genes were mainly enriched in fatty acid metabolism, and the PPARA signaling pathway was found to be over-activated and involved in the regulation of metabolic reprogramming of diabetic myocardial I/RI.
Conclusion
Metabolic reprogramming of diabetic myocardial I/RI may be the main cause of increased myocardial vulnerability. The number of myocardial subtype Cluster 0 increased significantly, and PPARA PPARA is a ligand-activated receptor of the nuclear hormone receptor family that plays a central regulatory role in lipid metabolism. signaling pathway activation may be a potential mechanism for reprogramming metabolism in diabetic myocardium.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


