Evaluation of the Choroid Plexus Epithelium Inflammation TLR4/NF-κB/NKCC1 Signal Pathway Activation in the Development of Hydrocephalus
Abstract
Background
Hydrocephalus is characterized by secretion, circulation, and absorption disorder of cerebrospinal fluid (CSF) with high morbidity and complication rate. The relationship between inflammation and abnormal secretion of CSF by choroid plexus epithelium (CPE) had received more attention. In this study, we aim to detect the role of Toll-like receptor 4/nuclear factor-kappa B/Na+/K+/2Cl-cotransporter 1(TLR4/NF-κB/NKCC1) signal pathway in the development of hydrocephalus.
Method
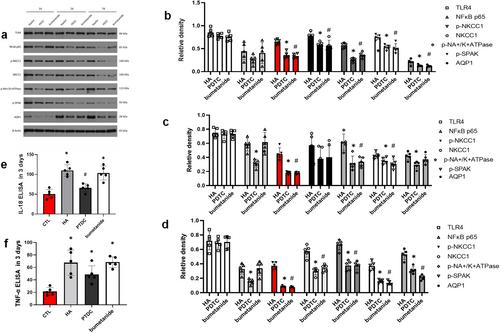
Hydrocephalus was induced in adult rats (8 weeks) by intracisternal kaolin injection, then pyrrolidinedithiocarbamate (PDTC) and bumetanide were administrated to the rats mode. Then the rat model was evaluated, and ventricular volume was calculated at different time points. Then CPE, cortex, preventricular tissue, and CSF were obtained. Protein expressions of TLR-4, NKCC/serine–threonine STE20/SPS1-related, proline-alanine-rich kinase (SPAK), pNKCC1, pSPAK, GFAP, AQP1, and AQP4 were measured by RT-PCR, western blot, and immunofluorescence (IF) stains in CPE, respectively.
Result
Our data showed that inflammation factors tumor necrosis factor-(TNF-α), interleukin 18(IL-18), and glial fibrillary acidic protein (GFAP) concentrations were significantly higher in the model group than in controls. The TLR4/NF-κB/NKCC1 signal pathway were actived by NF-κB-p65, NKCC1, pNKCC1- pSPAK complex, and Aquaporin1 (AQP1) high expression. PDTC and bumetanide use can help regular TLR4/NF-κB/NKCC1 expression and reduced AQP1 expression by down-regulate NF-B-p65 and inhibiting NKCC1, respectively. As a result, the treatment groups alleviated CPE abnormal secretion and ventricle enlargement.
Conclusion
These results confirmed that the inflammatory reaction contributes TLR4/NF-κB/NKCC1 mediated CPE abnormal secretion and consequent hydrocephalus. Regulation of TLR4/NF-κB/NKCC1 and AQP1 can prevent this process. Our study provides a strong rationale for further exploring alleviating CPE abnormal secretion as a therapeutic perspective of hydrocephalus.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


