Zn2+ ions improve the fidelity of metal-mediated primer extension while suppressing intrinsic and Mn2+-induced mutagenic effects by DNA polymerases†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
While Mn2+ ions are well-established for reducing the fidelity of DNA polymerases, leading to the misincorporation of nucleotides, our investigation of the effects of metal ions revealed a contrasting role of Zn2+. Here, we demonstrate that Zn2+ ions enhance the fidelity of DNA polymerases (the 3′ → 5′ exonuclease-deficient Klenow fragment and Taq DNA polymerase) by suppressing misincorporation during primer extension reactions. Remarkably, Zn2+ ions inhibit both intrinsic misincorporation and Mn2+-induced misincorporation of nucleotides. Furthermore, Zn2+ ions also effectively suppressed misincorporation during metal-mediated primer extension reactions, which involved forming Ag+ and Hg2+ ion-mediated base pairs. These findings suggest that Zn2+ ions inhibit both intrinsic and Mn2+-induced mismatched base pair formation. Consequently, the combined use of Mn2+ and Zn2+ ions may offer a strategy for precisely regulating the fidelity of DNA polymerases. Remarkably, Zn2+ ions even suppress misincorporation in primer extension reactions that rely on metal-mediated base pairs, and conversely, this suggests that DNA polymerases recognize metal-mediated base pairs such as T-Hg2+-T, C-Ag+-A, and C-Ag+-T as relatively stable base pairs. These results imply that Zn2+ ions may also enhance the fidelity of DNA polymerases when incorporating non-canonical nucleobases, potentially paving the way for the expansion of the genetic alphabet.
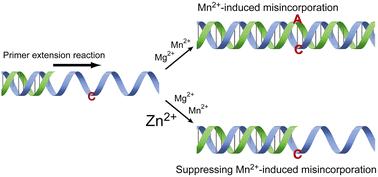
Zn2+ 离子提高了金属介导的引物延伸的保真度,同时抑制了 DNA 聚合酶的内在诱变效应和 Mn2+ 诱导的诱变效应。
Mn2+ 离子可降低 DNA 聚合酶的保真度,导致核苷酸错结合,这一点已得到公认,而我们对金属离子作用的研究则发现了 Zn2+ 的相反作用。在这里,我们证明了 Zn2+ 离子通过抑制引物延伸反应中的核苷酸错结合,提高了 DNA 聚合酶(3' → 5' 外切酶缺陷的 Klenow 片段和 Taq DNA 聚合酶)的保真度。值得注意的是,Zn2+ 离子能抑制核苷酸的固有错结合和 Mn2+ 诱导的错结合。此外,Zn2+ 离子还能有效抑制金属介导的引物延伸反应中的错结合,该反应涉及形成 Ag+ 和 Hg2+ 离子介导的碱基对。这些发现表明,Zn2+ 离子可抑制固有碱基对和 Mn2+ 诱导的错配碱基对的形成。因此,结合使用 Mn2+ 和 Zn2+ 离子可为精确调节 DNA 聚合酶的保真度提供一种策略。值得注意的是,在依赖金属介导的碱基对的引物延伸反应中,Zn2+ 离子甚至能抑制错结合,反之,这表明 DNA 聚合酶能识别金属介导的碱基对,如 T-Hg2+-T、C-Ag+-A 和 C-Ag+-T 等相对稳定的碱基对。这些结果表明,Zn2+ 离子也可能会提高 DNA 聚合酶结合非规范核碱基时的保真度,从而有可能为扩展遗传字母表铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


