Fast dehydration reduces bundle sheath conductance in C4 maize and sorghum
IF 8.1
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract

快速脱水会降低 C4 玉米和高粱的束鞘传导性
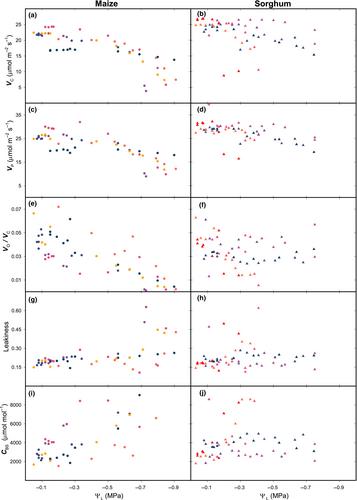
摘要 面对人为变暖,干旱对粮食生产的威胁日益严重。C4 植物有望解决这一威胁。C4 植物叶片具有一种生化二氧化碳浓缩机制,可在两个部分隔离的隔室(叶肉和叶鞘)之间交换代谢产物,这使其在炎热的气候条件下具有高生产潜力,提高了水分利用效率。然而,当C4叶片脱水时,光合作用会急剧下降。本文探讨了这种下降背后的生理机制。在快速脱水实验中,我们测量了叶片交换气体中水和二氧化碳的通量和同位素组成,并利用新型生化模型和弹性分析对结果进行了解释。我们的研究结果表明,虽然在脱水过程中叶肉和叶束鞘的二氧化碳供应持续存在,但叶束鞘-叶肉界面的二氧化碳传导却有所下降。我们将此解释为导致了细胞间代谢物交换的减慢--这是 C4 光合作用的一个基本特征。这将阻碍向束鞘提供还原力,导致甘油磷酸积累和 Rubisco 羧化的反馈抑制。这种快速敏感性与 C4 植物所采用的应对策略的有效性之间的相互作用,可能是其竞争表现的一个被忽视的驱动因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

New Phytologist
生物-植物科学
自引率
5.30%
发文量
728
期刊介绍:
New Phytologist is an international electronic journal published 24 times a year. It is owned by the New Phytologist Foundation, a non-profit-making charitable organization dedicated to promoting plant science. The journal publishes excellent, novel, rigorous, and timely research and scholarship in plant science and its applications. The articles cover topics in five sections: Physiology & Development, Environment, Interaction, Evolution, and Transformative Plant Biotechnology. These sections encompass intracellular processes, global environmental change, and encourage cross-disciplinary approaches. The journal recognizes the use of techniques from molecular and cell biology, functional genomics, modeling, and system-based approaches in plant science. Abstracting and Indexing Information for New Phytologist includes Academic Search, AgBiotech News & Information, Agroforestry Abstracts, Biochemistry & Biophysics Citation Index, Botanical Pesticides, CAB Abstracts®, Environment Index, Global Health, and Plant Breeding Abstracts, and others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


