Understanding the Different Roles of Adsorbed Oxygen and Lattice Oxygen Species in the Distinct Catalytic Performance of Metal Oxides for o-Xylene Oxidation
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
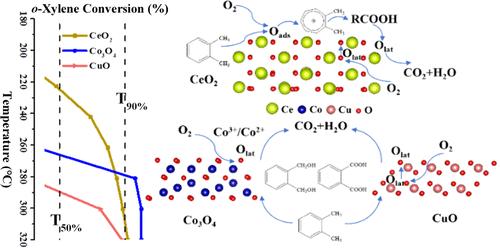
Metal oxides have always been considered as promising non-noble metal catalysts for VOC elimination and generally show distinct performance based on their reactive oxygen species (ROS). This work originally investigated the roles of adsorbed oxygen (Oads) and lattice oxygen (Olat) species in the catalytic oxidation of o-xylene. A series of metal oxide catalysts were synthesized through the pyrolysis of MOF precursors. The CeO2 catalyst showed performance superior to that of other metal oxides at lower temperature, while the Co3O4 catalyst had advantages over other metal oxides in the complete oxidation of o-xylene and CO2 generation but also exhibited a larger decrease of o-xylene conversion with the drop of O2 concentration. The o-xylene-TPD and o-xylene-TPSR (18O2/He) profiles of CeO2, Co3O4, and CuO indicated that the Oads served as the primary ROS of CeO2 and that the Olat played decisive roles in the cases of Co3O4 and CuO. Notably, the surface Olat of Co3O4 could be rapidly and completely replenished by gaseous oxygen, relying more on gaseous oxygen compared to CuO. Furthermore, in situ DRIFTS studies and DFT calculations disclosed the interactions of different ROS with o-xylene. The Oads on the CeO2 surface favored the adsorption and cleavage oxidation of the aromatic ring at lower temperature, while the Olat on the Co3O4 and CuO surface preferentially oxidized methyl groups and favored the oxidation of intermediates. Therefore, the different interactions with o-xylene and replenishment of ROS are responsible for the performance differences of CeO2, Co3O4, and CuO in the catalytic oxidation of o-xylene. This work might provide insights into the catalytic mechanism of metal oxides and benefit the design and application of efficient metal oxide catalysts for VOC elimination.
了解吸附氧和晶格氧在邻二甲苯氧化金属氧化物催化性能差异中的不同作用
金属氧化物一直被认为是很有前途的消除挥发性有机化合物的非贵金属催化剂,并且通常根据其活性氧种类(ROS)显示出不同的性能。这项工作最初研究了吸附氧(Oads)和晶格氧(Olat)物种在邻二甲苯催化氧化中的作用。通过热解 MOF 前体合成了一系列金属氧化物催化剂。CeO2 催化剂在较低温度下的性能优于其他金属氧化物,而 Co3O4 催化剂在完全氧化邻二甲苯和生成 CO2 方面优于其他金属氧化物,但随着 O2 浓度的降低,邻二甲苯的转化率也有较大的下降。CeO2、Co3O4 和 CuO 的邻二甲苯-TPD 和邻二甲苯-TPSR (18O2/He) 曲线表明,Oads 是 CeO2 的主要 ROS,而 Olat 在 Co3O4 和 CuO 中起着决定性作用。值得注意的是,Co3O4 的表面奥拉特可以被气态氧快速、完全地补充,与 CuO 相比更依赖于气态氧。此外,原位 DRIFTS 研究和 DFT 计算揭示了不同 ROS 与邻二甲苯的相互作用。CeO2 表面的 Oads 在较低温度下有利于芳香环的吸附和裂解氧化,而 Co3O4 和 CuO 表面的 Olat 则优先氧化甲基,有利于中间产物的氧化。因此,与邻二甲苯的不同相互作用以及 ROS 的补充是 CeO2、Co3O4 和 CuO 在催化氧化邻二甲苯过程中性能差异的原因。这项工作可能有助于深入了解金属氧化物的催化机理,并有利于设计和应用高效的金属氧化物催化剂来消除挥发性有机化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


